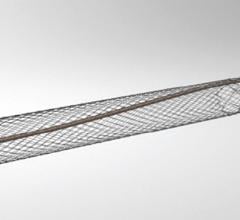
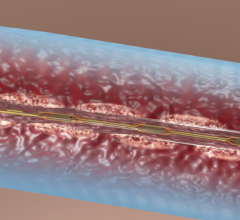
October 9, 2007 - OmniSonics Medical Technologies Inc. has received clearance from the FDA to market its catheter based OmniWave Endovascular System for the infusion of physician specified fluids, including thrombolytics, and for the removal of thrombus in the peripheral vasculature, as the company targets the deep vein thrombosis (DVT) market.
The OmniWave Endovascular System has a minimally invasive catheter-based technology that delivers low-power, transverse ultrasonic energy designed to remove thrombus quickly, safely and effectively.
The device treats DVT, which affects approximately 2 million people in the U.S. every year, and acute limb ischemia, which affects over 250,000 people in the U.S. every year.
For more information: www.omnisonics.com


 November 21, 2022
November 21, 2022