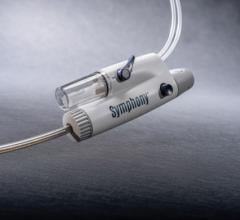
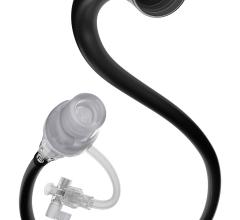
The latest in thrombus removal devices from Possis has received FDA clearance — the new AngioJet Ultra Thrombectomy System is the next-generation, re-engineered version of the company’s AngioJet Rheolytic Thrombectomy System.
AngioJet is marketed for blood clot removal (thrombectomy) from arterial and venous blood vessels. Possis says the new Ultra System features a simple and fast setup process, the flexibility to use a broad range of catheters, a sleeker design, lighter weight, and handling improvements that make it significantly easier to maneuver than the previous AngioJet drive unit.
Possis will conduct market evaluations of the Ultra System at key sites throughout the U.S. A full-market release is anticipated by Summer 2007.
AngioJet Systems are installed in 95 percent of top coronary labs in the U.S., according to a company release, with more than 1,700 systems are installed across the country. Possis currently estimates its total realizable market opportunity for AngioJet thrombectomy in the U.S. at $440 million, increasing to $675 million by 2010.


 January 19, 2026
January 19, 2026