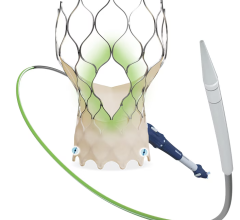
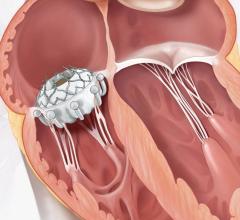
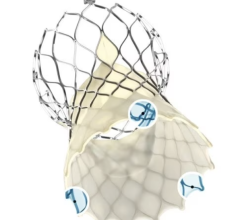
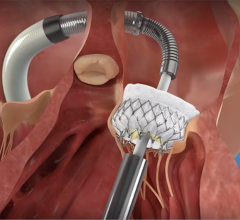
Edwards Lifesciences Corp. announced U.S. Food and Drug Administration (FDA) approval to expand use of the Edwards Sapien XT transcatheter heart valve for pulmonic valve replacement procedures. The approval enables the treatment of adult and pediatric patients who suffer from either a narrowed pulmonary valve or moderate or greater pulmonary regurgitation caused by congenital heart disease.
"U.S. approval of the Sapien XT valve for pulmonic procedures provides an important, minimally invasive treatment option for a small group of patients who typically face the burden of multiple open-heart surgeries, oftentimes beginning at birth or during childhood. As risks increase with each open-heart surgery, a non-surgical option can help them receive treatment, recover and return to normal activities sooner," said Larry L. Wood, Edwards' corporate vice president, transcatheter heart valves.
FDA approval of the Edwards Sapien XT valve for pulmonic procedures was supported by data from the multicenter COMPASSION clinical trial and additional clinical data from Europe. As previously indicated, the commercial opportunity related to this approval is small and is factored into 2016 financial expectations.
For more information: www.edwards.com


 July 08, 2024
July 08, 2024