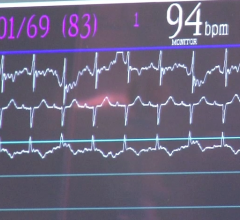
March 6, 2007 — Federal health advisors recommended in a 9-2 vote that the FDA not approve the Chronicle Implantable Hemodynamic Monitor from Medtronic, a device intended to detect worsening heart failure. A study of the device by the manufacturer suggested it is not effective.
FDA is not required to follow its advisory committees’ advice but does so most of the time.
The device functions by taking heart rate, temperature, pressure and patient activity measurements. The data can be downloaded by doctors in the office or securely transmitted to the Web for viewing.
Medtronic is studying a defibrillator that incorporates the monitoring technology.
"We continue to believe in the benefit of this sound technology and intend to work in close collaboration with the FDA to define the appropriate path for approval," said Dr. David M. Steinhaus, vice president and medical director of Medtronic’s cardiac rhythm disease management.
___

 January 13, 2026
January 13, 2026