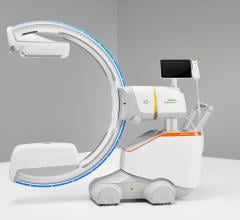
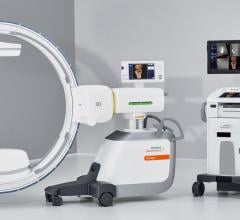
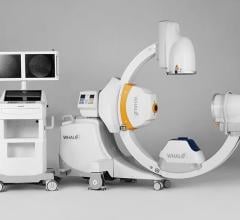
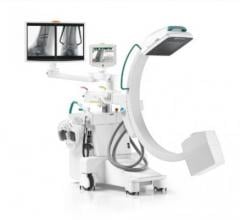
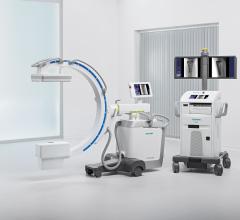
May 5, 2008 - The FDA has notified GE Healthcare's Surgery Business that the company has satisfied the criteria in the January 2007 consent decree required to resume operations, and can distribute the OEC 9900 Elite C-arm.
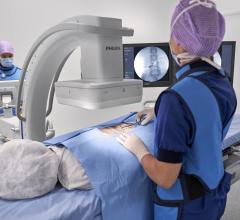
The 9900, a fluoroscopy device that uses X-rays to reveal real-time imagery of a patient's internal structure, is the first product to receive manufacturing and shipping authorization. More than 300 of these units will be shipped to customers in the first 10 days of operations.
After an August 2006 FDA inspection of GE facilities in Salt Lake City and Lawrence, MA, the FDA required that the company suspend C-arm manufacturing and distribution until it met FDA quality management criteria, specific regulations and technical improvements.
Since that time, GE has taken the necessary steps to improve the 9900 and 9800 models, and subsequently filed a new 510(k) submission for the 9900 Elite C-arm "because the company felt it had made enough modifications to the system to warrant a new application," according to Peter McCabe, president of CEO of GE Healthcare Surgery Business. GE addressed technical issues with the 9900 first because that system is the company's flagship C-arm. The 9800 model is also undergoing the same process, but the company has not said if it will need to file a 510(k) for that system.
For more information: www.gehealthcare.com

 June 20, 2024
June 20, 2024