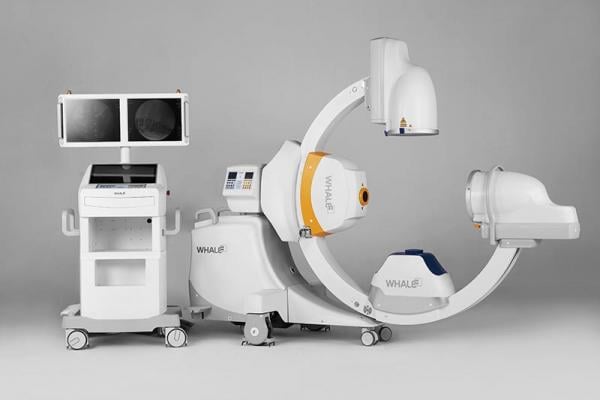
January 9, 2017 — Whale Imaging Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the G-Arm Duo. The Duo is the next generation of the G-Arm, with major improvements including axial tilt and greater table access.
The new system was designed to help the user improve accuracy while reducing time, infection and radiation. Engineers at Whale made the Duo compatible with all surgical tables, and added many new features including axial tilt. Whale’s unique ability to fire two X-ray beams asynchronously provides the customer with bi-lateral video and images, giving the user a more comprehensive picture than a traditional C-arm. G-Arm utilizes this X-Beam technology, as well as iC-Clear algorithms to provide the best image possible, using the smallest dose of radiation.
For more information: www.whaleimaging.com


 October 24, 2025
October 24, 2025 









