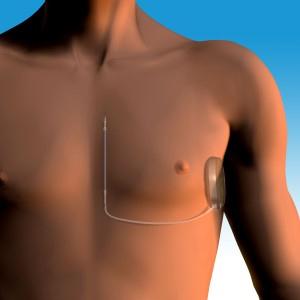
May 1, 2012 — Cameron Health Inc. announced that the U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel voted 7-1 that sufficient data exists demonstrating the efficacy and safety of the S-ICD (subcutaneous-implantable cardioverter defibrillator) system for the treatment of sudden cardiac arrest (SCA).
"We are pleased with the panel's strong recommendation for approval of the S-ICD system, the world's first and only completely subcutaneous ICD for the treatment of sudden cardiac arrest," said Kevin Hykes, president and CEO of Cameron Health. "This represents another important step on the path toward FDA approval of the S-ICD system and its availability to physicians and their patients at risk for sudden cardiac arrest."
Cameron Health submitted a premarket approval application (PMA) in December 2011 based on data from a 330-patient pivotal investigational device exemption (IDE) clinical study that evaluated the safety and efficacy of the S-ICD system in patients at risk of SCA.
"The S-ICD system is a breakthrough technology that holds promise as a new alternative for treating patients at risk for sudden cardiac arrest," said Michael R. Gold, professor of medicine and chief of cardiology at the Medical University of South Carolina. "The S-ICD system provides the same defibrillation protection as conventional ICDs, but without the serious complications associated with leads that reside in the heart and blood vessels."
On March 8, 2012, Boston Scientific Corp. announced that it would exercise its option to acquire Cameron Health. Closing of the transaction is subject to customary conditions, including relevant antitrust clearance, and is expected to occur in the second or third quarter of 2012.
For more information: www.cameronhealth.com


 January 13, 2026
January 13, 2026 









