
The U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel is set to review data and offer recommendations concerning the final approval of Abbott’s Absorb fully bioresorbable stent and the AngelMed Guardian System. These devices are both the first of their kind to face FDA review and a positive recommendation may lead to paradigm shifts in how coronary lesions are treated and how high-risk patients are monitored for the onset of heart attacks.
The panel will hold its public meeting March 15-6, 2016, from 8 a.m. to 6 p.m. at the Holiday Inn Gaithersburg, Two Montgomery Village Ave., Gaithersburg, Md. The panel’s purpose is to review the available scientific evidence regarding new cardiovascular technologies and provide advice and recommendations to the agency on FDA's regulatory issues. The FDA is not required to follow the panel’s recommendations, but it usually does. A positive set of recommendations may mean these devices could see a final market decision later this year.
On March 15, the committee will discuss, make recommendations and vote on information related to the premarket approval application (PMA) for the Absorb GT1 Bioresorbable Vascular Scaffold (BVS) System from Abbott Vascular. The Absorb BVS System is a temporary scaffold that will fully resorb over a period of about two years. The PMA is seeking an indication for improving coronary luminal diameter in patients with ischemic heart disease due to de novo native coronary artery lesions (length ≤ 24 mm) with a reference vessel diameter of ≥ 2.5 and ≤ 3.75 mm.
“Abbott looks forward to providing the panel with a robust view of the clinical trial results for Absorb and patient experiences among the more than 125,000 patients who have been treated with this naturally dissolving device, in more than 100 countries,” said Abbott spokesman Steven Kelly.
The Absorb has become a popular device in Europe since the first generation device’s market launch in 2012. Abbott’s latest generation Absorb GT1 and improved GlideTrack delivery system gained Eurpean CE mark approval in May 2015.
Results from the ABSORB II pivotal FDA clinical trial showed the Absorb everolimus-eluting stent was non-inferior after one year compared to Abbott's Xience V, the best current generation metallic drug-eluting stent (DES) on the market. The trial compared the two stents in patients with coronary artery disease and measured target lesion failure (TLF) was the primary endpoint. Findings from the ABSORB III trial were presented as one of the key late-breaking studies at the 2015 Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium last fall.
Cardiology societies, market analysts and most cardiologists believe drug-eluting bioresorbable stents may replace the current gold standard of drug-eluting metallic stents in the coming years. The new devices eliminate the use of permanent implants and clinical evidence indicates treated vessels return to normal function after the stent dissolves, allowing vasodilation and vasoconstriction.
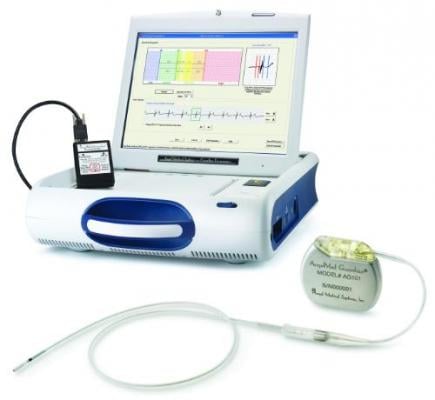
On March 16, the committee will discuss, make recommendations and vote on information related to the PMA application for the AngelMed Guardian System from Angel Medical Systems Inc. The Guardian System is an implantable cardiac monitor intended to alert patients to ST segment shifts that indicate coronary ischemia due to a blockage of the coronary vessels. For patients with prior acute coronary syndrome events who are at risk for recurrent heart attacks, the system is designed to reduce the symptom-to-door times. These times are currently about two to three hours, and the longer it takes to revacularize the blockages, the poorer the patient outcomes. The system serves as an early warning system, alerting patients that they need to seek immediately medical attention, rather than patients waiting to see if symptoms subside or get worse.
For more information: www.fda.gov/AdvisoryCommittees/Calendar/ucm481684.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery


 November 12, 2025
November 12, 2025 









