Impella Improves Survival and Heart Recovery
November 28, 2023 — Abiomed, part of Johnson & Johnson MedTech[1], announces the first patient in the world has been enrolled in the landmark RECOVER IV randomized controlled trial (RCT). The on-label, two-arm trial will randomize 548 patients to assess whether Impella support prior to percutaneous coronary intervention (PCI) is superior to PCI without Impella in patients with acute myocardial infarction (AMI) cardiogenic shock.
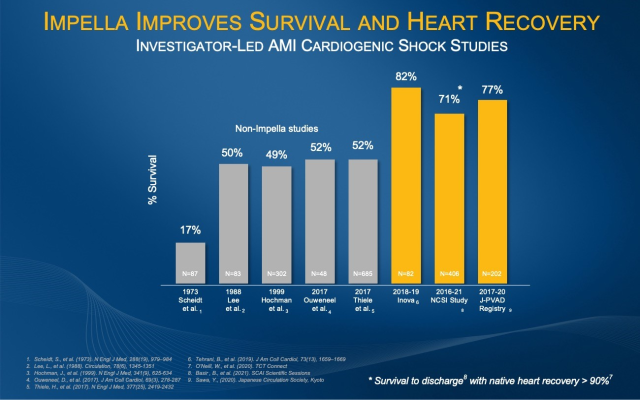
Impella is the only mechanical circulatory support device for the treatment of AMI cardiogenic shock with premarket approval from the United States Food and Drug Administration. Multiples studies, including the Inova Study[2], the National Cardiogenic Shock Initiative (NCSI) Study[3] and the Japanese J-PVAD Study[4], have demonstrated significant improvement in AMI cardiogenic shock survival rates when Impella and best practices are implemented, including the use of Impella prior to PCI and identifying shock early.
“Fifteen years of clinical studies have helped us establish best practices and shown that Impella increases survival and heart recovery in AMI cardiogenic shock patients,” said Chuck Simonton, MD, Abiomed’s chief medical officer. “The design of RECOVER IV, which incorporates those best practices, is intended to further validate the benefits of Impella utilization in AMI cardiogenic shock patients and provide evidence to achieve a Class 1 guideline recommendation.”
This first patient was enrolled at New Mexico Heart Institute by Mark Bieniarz, MD[5], an interventional cardiologist and the principal investigator for RECOVER IV at New Mexico Heart Institute. RECOVER IV is the first trial within the field of AMI cardiogenic shock to use the U.S. Food and Drug Administration’s (FDA) exception from informed consent (EFIC) protocol, an emergency research regulation. Since AMI cardiogenic shock patients are often too sick to provide consent, it has been difficult to study this patient population. Emergency research regulations require study investigators to consult and disclose information about the study with representatives from the community where the research will take place. EFIC allows for patients to be enrolled prior to obtaining traditional consent.
“We are thrilled to be the first site to enroll a patient into the historic RECOVER IV trial,” said Dr. Bieniarz. “The EFIC process provides us a pathway to successfully enroll patients and compare outcomes in patients who receive Impella support for acute myocardial infarction cardiogenic shock and those patients who do not receive Impella support for the same condition.”
The primary endpoint of RECOVER IV is all-cause mortality at 30 days. Secondary endpoints include major adverse cardiovascular and cerebrovascular events at 30 days, days alive out of the hospital at six months, recovery of left ventricular (LV) function, need for durable ventricular assist device or heart transplant, and health-related quality of life as measured by responses to the Kansas City Cardiomyopathy Questionnaire at one year. Abiomed’s goal in conducting the RECOVER IV RCT is to achieve AMI cardiogenic shock Class I guideline recommendations for Impella use in AMI cardiogenic shock. (See figure 2).
For more information about RECOVER IV, click here.
For more information: www.abiomed.com
References:
[1]Abiomed, Inc. is part of Johnson & Johnson MedTech, which comprises the surgery, orthopedics, vision and interventional solutions businesses within Johnson & Johnson's MedTech segment.
[2] Tehrani, B., et al. (2019). J Am Coll Cardiol, 73(13), 1659-1669
[3] Basir, B., et al. (2021). SCAI Scientific Sessions
[4] Sawa, Y., (2020). Japanese Circulation Society, Kyoto
5Dr. Mark Bieniarz serves as PI for the RECOVER IV RCT at New Mexico Heart Institute. He is not compensated for his involvement with this research study or press release.


 February 03, 2026
February 03, 2026 









