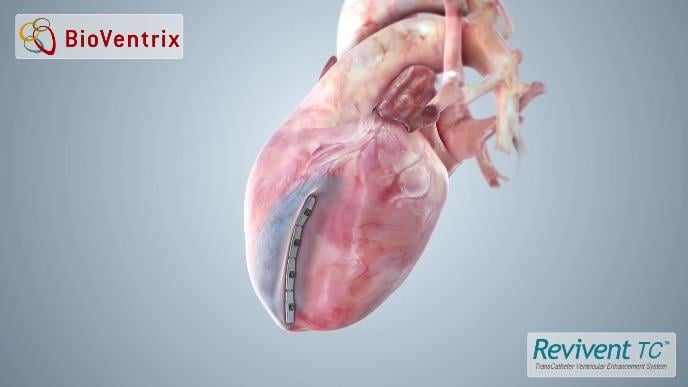
October 17, 2017 — BioVentrix Inc. recently announced enrollment of the first patient in the U.S. arm of the ALIVE pivotal clinical trial. The trial is designed to demonstrate the safety and effectiveness of the Revivent TC TransCatheter Ventricular Enhancement System, a hybrid closed-chest procedure to treat patients suffering from heart failure symptoms related to cardiomyopathy. BioVentrix previously received investigational device exemption (IDE) approval for the study from the U.S. Food and Drug Administration (FDA).
The Less Invasive Ventricular Enhancement (LIVE) procedure was performed at the University of Pittsburgh Medical Center (UPMC) Heart and Vascular Institute by interventional cardiologist Catalin Toma, M.D., and cardiothoracic surgeon Christopher Sciortino, M.D. The device was successfully implanted with three micro-anchor pairs in a 42-year-old female patient suffering from ischemic heart failure.
"UPMC remains at the forefront of implementing promising less invasive therapies to address the need for better heart failure treatment. We are pleased to be the first U.S. center to implant the Revivent TC System as part of the ALIVE clinical trial. The procedure aims to reshape the left ventricle, decrease the left ventricular end systolic volume index (LVESVI), and increase the ejection fraction (EF). The patient was discharged shortly after the procedure and is recovering well," said Toma.
"The LIVE procedure is less invasive and less traumatic than conventional surgical reconstruction. It expands the patient population that can be treated by reducing the procedural risk. The patient was able to undergo this procedure without having to perform a sternotomy or employ cardiopulmonary bypass," said Sciortino.
The ALIVE trial plans to enroll 120 patients at up to 20 sites in the U.S. and U.K. with a primary endpoint analysis at one year. The trial endpoints include positive effects on volume reduction, ejection fraction, quality of life (QOL), New York Heart Association (NYHA) Class, six-minute walk test and rehospitalization. Readmission rates following heart failure hospitalization remain high using standard therapies, with ≥ 50 percent of patients readmitted to the hospital within six months of discharge. Left ventricular volume reduction has been shown to significantly impact short and long-term survival rates.1 Annually, over 1 million patients are hospitalized with a primary diagnosis of heart failure, accounting for a total Medicare expenditure exceeding $17 billion.2
The Revivent TC System is approved for sale in Europe; it is not approved for sale in the United States.
For more information: www.bioventrix.com
References
1. White, HD, et al; Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation; 1987 Jul;76 (1):44-51.
2. Desai et al., Rehospitalization for heart failure. Circulation. 24 July 2012 (126:501-506).


 November 14, 2025
November 14, 2025 









