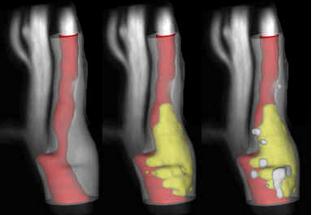
MRI-PlaqueView's 3-D visualization of carotid plaque morphology and composition.
July 13, 2012 — GE Healthcare and VPDiagnostics Inc. jointly announced today their co-marketing agreement for the VPDiagnostics’ MRI-PlaqueView software for carotid artery atherosclerosis analysis. The MRI-PlaqueView software is compatible with carotid magnetic resonance imaging (MRI) data acquired from GE 3.0T MRI systems (HDxt and Discovery MR750). The collaboration will aim to develop optimized carotid imaging protocols and future co-developments on new techniques.
Carotid atherosclerosis may be responsible for as much as one-third of all strokes. To better gauge risk of stroke from carotid atherosclerosis, clinicians are beginning to look beyond traditional risk factors such as carotid artery narrowing (stenosis) to identify high-risk features of the atherosclerotic plaque itself. The GE 3.0T MRI systems and dedicated carotid coil enables high-resolution multi-contrast imaging of carotid lumens, vessel walls and atherosclerotic plaques, while the MRI PlaqueView software from VPDiagnostics provides analysis of these images to delineate various plaque components such as vessel wall, calcified and soft plaques, and help provide a better assessment of the plaque characteristics.
For more information: www.gehealthcare.com


 August 17, 2023
August 17, 2023 








