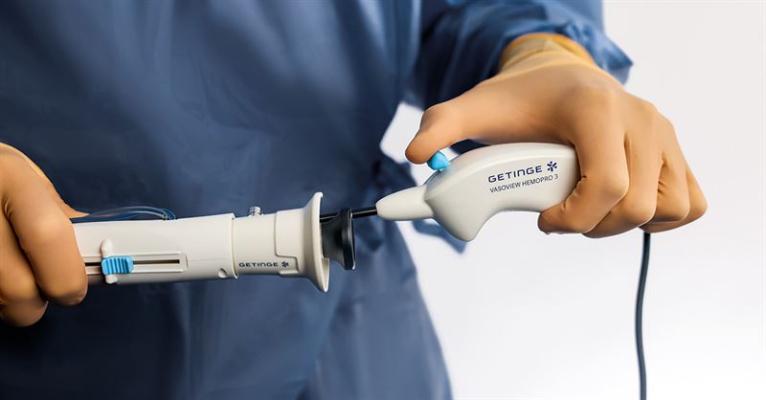
Getinge has announced the U.S. Food and Drug Administration’s (FDA) 510(k) clearance of the Vasoview Hemopro 3, the latest addition to the medtech company’s endoscopic vessel harvesting (EVH) solutions. Image courtesy: Getinge
March 12, 2024 — Getinge has announced the U.S. Food and Drug Administration’s (FDA) 510(k) clearance of the Vasoview Hemopro 3, the latest addition to the medtech company’s endoscopic vessel harvesting (EVH) solutions. Designed with customer centricity top of mind, the newcomer is expected to offer enhanced procedural efficiency in healthcare from the upcoming launch, according to a written statement released March 8 by the Gothenburg, Sweden-based company.
The statement added that receiving the FDA 510(k) clearance for Vasoview Hemopro 3 marks a significant milestone for Getinge and underscores the effort to comply with the highest safety and effectiveness standards, emphasizing the medtech company’s commitment to advancing medtech.
“EVH is a therapy significantly helping patients towards faster recovery by minimizing surgical trauma and reducing post-operative pain,” said Elin Frostehav, President Acute Care Therapies at Getinge. “By offering solutions together with extensive training, we aim to continue help facilitating the widespread adoption of this essential therapy. The new generation of Vasoview – Hemopro 3 – symbolizes our dedication to elevating healthcare standards together with our customers,” Frostehav added.
The culmination of extensive market research and advisory boards conducted in partnership with clinicians within the EVH field, Vasoview Hemopro 3 represents collaborative innovation, reported the company. It further noted that feedback from the studies has been central in shaping the product, focusing on improvements that enhance harvester efficiency and patient outcomes. Advancements include enhanced smoke evacuation, regulated energy control, ergonomic game controller style handle, and an integrated cable.
Getinge is preparing for the product launch of Vasoview Hemopro 3 to occur in the United States during the third quarter of 2024, while registration in additional key markets is underway.


 January 15, 2026
January 15, 2026 









