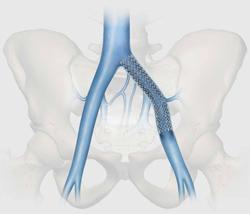
April 15, 2013 — Cook Medical has launched the VIVO clinical research study to evaluate the safety and effectiveness of the Zilver Vena Venous Self-Expanding Stent in the treatment of symptomatic iliofemoral venous outflow obstruction. This disease is characterized by leg pain, throbbing, swelling and skin discoloration in the legs. The Zilver Vena venous stent was designed specifically for the dynamic environment of the iliofemoral veins.
The VIVO clinical research study, led by global principal Investigators Anthony J. Comerota from Jobst Vascular Institute in Toledo, Ohio, and Lawrence “Rusty” Hofmann from Stanford University, is a prospective, nonrandomized study that will enroll 243 patients at up to 30 participating sites in the United States and the Asia-Pacific region.
“This is an important trial which is intended to objectively assess the endovenous correction of symptomatic iliofemoral vein stenosis with a stent designed for this specific purpose,” said Anthony Comerota, M.D., one of the research leaders from Jobst Vascular Institute in Toledo, Ohio. “It is a privilege to be a part of this crucial prospective trial.”
The VIVO clinical research study will enroll adults 18 years and older who have leg pain that limits usual activities, swelling or skin discoloration in the leg or a healed or active lower leg ulcer. There are additional eligibility criteria for the study. The primary study results will be evaluated one year after stent placement, with patient follow-up through three years after stent placement.
For more information: www.cookmedical.com


 January 05, 2026
January 05, 2026 









