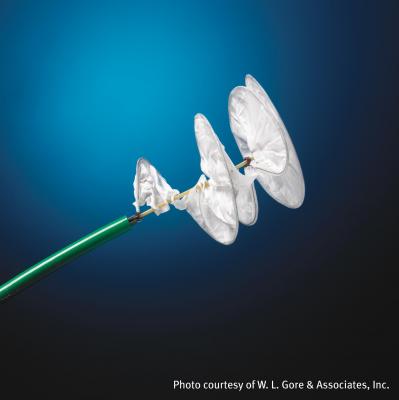
October 30, 2012 — W. L. Gore & Associates responded to initial results reported in St. Jude Medical Inc.’s RESPECT clinical trial. The RESPECT study investigated whether transcatheter closure of patent foramen ovale (PFO) using St. Jude’s Amplatzer PFO Occluder device is safe and effective compared to best medical therapy in the prevention of recurrent cryptogenic stroke. Gore is concurrently conducting its Gore REDUCE Clinical Study using both the Gore Helex Septal Occluder and, as reported earlier this week, the new Gore Septal Occluder, in patients with PFO and a history of cryptogenic stroke or imaging-confirmed transient ischemic attack (TIA).
“Our commitment is to patients suffering from cryptogenic strokes and bringing them viable and beneficial treatment options,” said Stuart Broyles, Ph.D., associate with the Gore medical division stroke business, “Our goal is to reduce recurrent stroke and improve the quality of life for patients. The RESPECT study data suggest closure therapy for PFO may be beneficial, but further research is required. Gore is committed to the pursuit of a PFO indication in the U.S. for the GORE Helex Septal Occluder and the Gore Septal Occluder. Worldwide, these Gore devices have a strong record of patient safety. We will continue to pursue the indication for our devices through the Gore REDUCE Clinical Study. Gore looks forward to further review of the RESPECT data in the coming weeks.”
For more information: www.clinical.goremedical.com/REDUCE


 January 05, 2026
January 05, 2026 









