January 8, 2018 —Guerbet announced that it has entered into an agreement under which it will acquire Israeli company Accurate Medical Therapeutics , which specializes in the development of microcatheters used in interventional radiology.
Accurate has developed a range of microcatheters for embolization procedures of tumors and vascular aneurysms. These products are currently being registered with the U.S. and European health authorities.
This range includes two series of microcatheters:
- The first series was designed and developed to propose an optimization of microcatheters intra-arterial navigation to interventional radiologists, even in case of tortuous vascular network and hardly accessible lesions; and
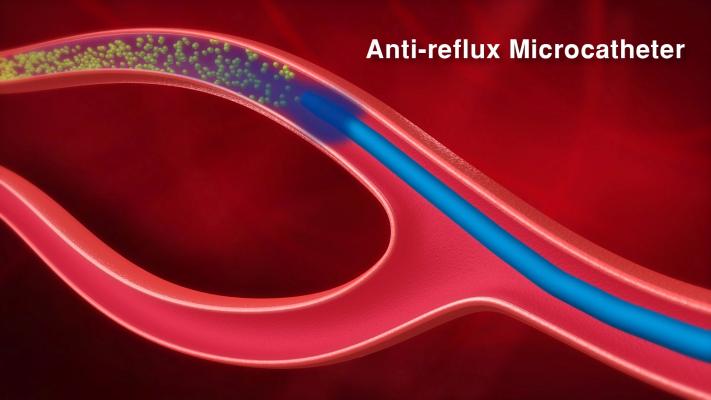
- The second series possess the same optimized navigation qualities, while also incorporating anti-reflux technology. This effect is achieved thanks to side holes located in the terminal part of the catheter, which create a fluid barrier preventing the reflux of embolization microspheres.
This new technology makes it possible to administer more embolization microspheres to the targeted treatment area while also preventing them from refluxing upstream to arteries irrigating healthy tissues (non-targeted areas that need to be preserved).
For more information: www.guerbet.com


 November 14, 2025
November 14, 2025 









