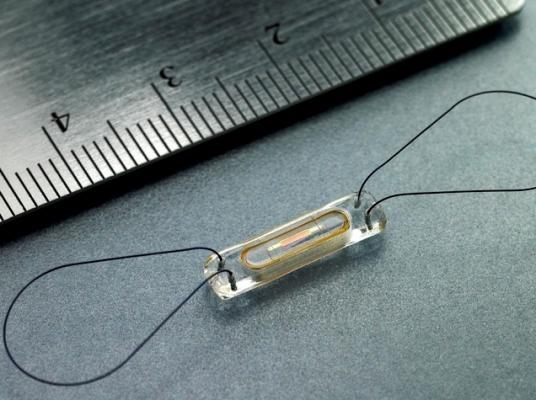
October 1, 2014 — On Sept. 24, Heart Hospital of Austin became the first facility in Texas to implant a new miniaturized, wireless monitoring sensor to manage heart failure (HF). The CardioMEMS HF system is the first and only U.S. Food and Drug Administration (FDA) -approved HF monitoring device that has been proven to significantly reduce hospital admissions when used by physicians to manage HF.
The CardioMEMS HF system features a sensor that is implanted in the pulmonary artery (PA) during a non-surgical procedure to directly measure PA pressure. An increase in PA pressure can appear before overt symptoms of heart failure, which include shortness of breath, abdominal swelling, ankle edema and weight gain. The principal concept behind the CardioMEMS HF system is to treat the patient's heart failure based on changes in their direct PA pressure assessment. The system transmits daily sensor readings to their healthcare providers, allowing for personalized and proactive management to reduce the likelihood of hospitalization.
"We are pleased to be the first hospital in Texas to offer this innovative device, allowing heart failure patients to better manage their conditions," said Kunjan A. Bhatt, M.D., clinical cardiologist at Heart Hospital of Austin and with Austin Heart. "With the CardioMEMS HF system, we can monitor patients' conditions around the clock and immediately address any issues that may arise, keeping patients out of the hospital and improving their quality of life."
Heart failure occurs when the heart is unable to pump enough blood to meet the body's demands. According to the Centers for Disease Control and Prevention (CDC), more than 5.1 million Americans have heart failure, with 670,000 new cases diagnosed each year. Patients with heart failure are frequently hospitalized, experience a reduced quality of life and face a higher risk of death.
The CardioMEMS sensor is designed to last the lifetime of the patient, and it does not require batteries. Once implanted, the wireless sensor sends pressure readings to an external patient electronic system. There is no pain or sensation for the patient during the readings. The system allows patients to transmit critical information about their heart failure status to a clinician on a regular basis, without the need for additional clinic or hospital visits. This provides clinicians with the ability to detect worsening heart failure sooner and adjust treatment to reduce the likelihood that the patient will need to be hospitalized.
Data from a clinical trial showed the CardioMEMS technology reduces heart failure hospital admissions by up to 37 percent. The CHAMPION trial studied the effectiveness of the CardioMEMS HF system in New York Heart Association (NYHA) class III heart failure patients who had been hospitalized for heart failure in the previous 12 months. Results of the trial demonstrated a statistically significant 28 percent reduction in the rate of heart failure hospitalizations at six months, and a 37 percent reduction in heart failure hospitalizations during an average follow-up duration of 15 months.
For more information: www.cardiomems.com


 February 03, 2026
February 03, 2026 









