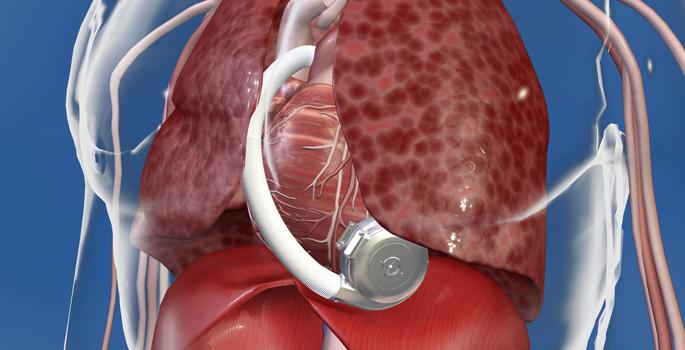
August 5, 2015 — HeartWare expanded the Class I recall of its ventricular assist device in mid-June due to concerns over damaged or failing device components. The three mid-June recalls are part of a series of five alerts related to the HeartWare VAD dating back to April.
The HeartWare VAD helps deliver blood from the heart to the rest of the body. It is used in patients who are at risk of death from end-stage left ventricular heart failure and who are waiting for a heart transplant. The system includes a pump implanted in the space around the heart (pericardium) and a controller that controls the speed and function of the pump.
On June 16, the company issued an alert related to damaged alignment guides and connector pins. According to the agency, the alignment guides in the power supply connector ports may wear down over time. This can cause the connection pins to become twisted or bent, and eventually prevent the patient from connecting the device controller to the system. An interruption in this electrical connection would cause the pump to stop, which could cause serious patient injury or death. The company has reported 33 instances of malfunction and one serious injury related to this problem.
On June 19, alerts were issued related to battery failure and damaged driveline connectors. According to the alert, the battery that powers an alarm in the controller may fail over time. If the battery fails, the alarm will not alert the patient in the event that both external power sources for the HVAD are disconnected.
Regarding the driveline connector, damage to the component may occur if the driveline is pulled too often with too much force. The driveline is a tube that connects the HVAD’s pump to the external controller and power source. Severe damage or disconnection of the driveline from the controller can cause electrical issues or pump stops that may lead to serious patient injury or death.
The firm has received a total of three reports, including one serious injury and two reports of death, related to damage to this issue. HeartWare said it would replace all defective controllers by the end of June 2016.
For more information: www.fda.gov


 February 03, 2026
February 03, 2026 









