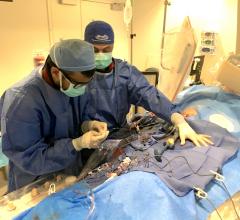
Study investigators Drs. Pedro Engel-Gonzalez and Gennaro Giustino from Henry Ford Health. Image courtesy of Hentry Ford Health
January 4, 2024 — Findings from a published case series research letter by the Henry Ford Health Structural Heart Disease team show that severe mitral stenosis, due to a build-up of calcium deposits in the mitral valve common in elderly patients, can be safely and successfully treated using Intravascular lithotripsy (IVL)-enabled percutaneous balloon mitral valvuloplasty. However, larger prospective studies in high-risk population are needed to confirm the findings.
These key takeaways from the research letter on IVL-facilitated valvuloplasty for severely calcified mitral valve stenosis are published in the Journal of the American College of Cardiology (JACC) Cardiovascular Interventions.
"We’ve developed a new strategy to treat our patients in the Detroit community and beyond," said corresponding author Pedro Engel Gonzalez, M.D., an interventional cardiologist and structural heart disease expert at Henry Ford Health. “We are looking forward to treating patients who have no other options for mitral stenosis diseased valves.”
IVL is a novel approach to lesion preparation of severely calcified plaques in coronary and peripheral vessels. Lithotripsy is delivered by vaporizing fluid to create an expanding bubble that generates sonic pressure waves that interact with arterial calcification.
In patients with severe mitral stenosis—which is the narrowing of the valve between the two left heart chambers that is a chronic degenerative process—who are not suitable for surgery or other transcatheter options, the IVL valvuloplasty is a possible option.
The Henry Ford team performed its first IVL-enabled mitral valvuloplasty back in 2019 and completed its first published case study. Now, the team has published its first case series report on the safety and efficacy of this procedure based on the 24 cases that have been performed so far.
The safety and efficacy results have Dr. Engel Gonzalez and the research team feeling very optimistic about the availability of this procedure to more patients going forward.
The research letter’s lead author, Gennaro Giustino, M.D., a Structural Heart Disease Fellow at Henry Ford Hospital, is also optimistic that these findings will lead to helping more people. “Patients with severely calcified mitral stenosis are often not candidates for conventional open-heart surgery,” said Dr. Giustino. “This minimally invasive technique pioneered at Henry Ford is a promising and safe treatment option for these patients to improve their cardiac symptoms and quality of life."
For more information: https://www.henryford.com/

 April 27, 2023
April 27, 2023