May 22, 2019 — Abiomed announced that the Impella CP with SmartAssist will be commercially available beginning at the 2019 Society for Cardiovascular Angiography & Interventions (SCAI) Scientific Sessions, May 19-22 in Las Vegas, through a controlled launch process at select sites. The majority of Impella CP heart pumps in the U.S. will be transitioned to SmartAssist over the next fiscal year.
Abiomed received U.S. Food and Drug Administration (FDA) approval for Impella CP with SmartAssist in March 2018. The device is designed to improve patient outcomes with advanced algorithms and simplified patient management. During a limited market release over the past year, more than 1,000 patients at 70 sites have been treated with the SmartAssist platform. These advances in Impella forward-flow heart pump technologies and software are designed to improve ease-of-use and patient management to optimize both survival and heart recovery:
-
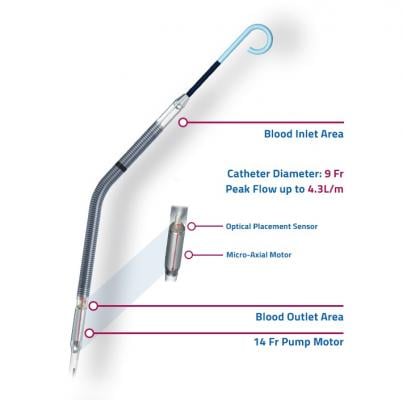
Weaning algorithms to optimize survival and native heart recovery: Real-time displays of critical hemodynamic metrics including left ventricular end-diastolic pressure (LVEDP), mean arterial pressure (MAP) and cardiac power output (CPO). Impella CP with SmartAssist is the only mechanical circulatory support device, according to Abiomed, that calculates and displays LVEDP, MAP and CPO.
-
Easier management and repositioning: SmartAssist sensor allows for precise positioning in the aorta and left ventricle and repositioning in the intensive care unit (ICU) without the need for catheterization lab or ultrasound imaging.
-
Greater hemodynamic support: Allows for sustained peak flows of up to 4.3L/minute (>85 percent of a normal cardiac cycle).
-
Faster, simplified Impella console setup: Less than 90 seconds.
“Access to real-time clinical data has allowed our team to identify best practices for patient management and weaning,” said Hiram Bezerra, interventional cardiologist, University Hospitals, Cleveland, and a participant in the limited market release. “Metrics such as LVEDP, MAP and CPO and the ability to monitor trends on the Impella console allow physicians to utilize this hemodynamic information to optimize heart recovery with objective and quantifiable data to enable clear communication within the shock team.”
The SmartAssist platform is complemented by Abiomed’s on-site and on-call support, including the Clinical Support Center, which provides 24/7 expert evaluation of Impella data and collaborative patient care to help improve outcomes.
Read the article "FDA Approves Abiomed Impella CP With SmartAssist and Optical Sensor"
For more information: www.abiomed.com


 November 14, 2025
November 14, 2025 









