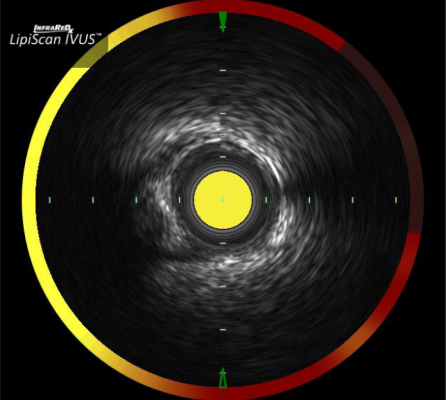
The Infraredx system combines IVUS with spectroscopy, which shows a halo ring of color, with yellow being plaque and red representing lipid core plaques based on the chemical composition.
April 28, 2015 — Infraredx Inc. announced the enrollment of 1,000 patients in the Lipid-Rich Plaque (LRP) Study. The LRP Study is a prospective, multi-center clinical trial designed to identify a correlation between lipid-rich plaques detected by Infraredx’s TVC Imaging System and the occurrence of a cardiac event within two years. The first-in-class dual-modality intravascular imaging system integrates near-infrared spectroscopy (NIRS) with intravascular ultrasound (IVUS) technology, allowing clinicians the ability to assess vessel structure and plaque composition. The TVC Imaging System is U.S. Food and Drug Administration (FDA)-approved to identify lipid-core plaques that may cause heart attacks. Identification of such plaques would be a major step toward the development of percutaneous coronary intervention (PCI) as a means to prevent coronary events.
Current PCI imaging technologies are limited in the information they can provide about non-flow limiting plaques that may be dangerous. The results of TVC imaging are presented in the form of a chemogram, an easy-to-read roadmap of cholesterol throughout the vessel scanned. Several prior studies in patients who have already experienced a coronary event have revealed a prominent signal detected by NIRS at the site of the culprit lesion. These studies led to the initiation of the LRP Study to test the hypothesis that a plaque with a large lipid core identified by NIRS imaging is a vulnerable plaque likely to cause a future coronary event. The goal is to prove that vulnerable plaques can be identified by NIRS and provide a target for personalized therapy to prevent coronary events.
“With 1,000 patients enrolled at 41 investigator sites across the United States and Europe, we are excited by the rapid progress of the LRP Study,” said Ron Waksman, M.D., principal investigator of the LRP Study. “Once complete, the LRP Study data could redefine the role of intravascular imaging and lay the groundwork for changing standard protocols for managing coronary artery disease.”
For more information: www.infraredx.com


 January 05, 2026
January 05, 2026 









