June 29, 2011 – To expand manufacturing operations and commercial infrastructure to support growing demand for the LipiScan IVUS (intravascular ultrasound) Coronary Imaging System, InfraReDx Inc. announced it raised $24.1 million from the sale of equity to existing InfraReDx shareholders.
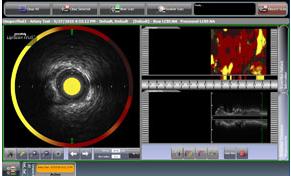
The system includes the world’s first and only cardiac catheter to combine intravascular ultrasound (IVUS) and near-infrared (NIR) spectroscopy to help cardiologists identify and characterize the plaques known to complicate stenting and suspected to cause most heart attacks. The funds raised will also be used to support ongoing and new clinical trials investigating expanded clinical applications of the LipiScan IVUS system.
InfraReDx launched the LipiScan IVUS system in September 2010 after receiving U.S. Food and Drug Administration (FDA) approval. The only FDA-cleared device for the detection of lipid core coronary plaques (LCP), the system is now being utilized in a growing number of cardiac centers across the United States.
In a single catheter pullback, the LipiScan IVUS provides physicians with a traditional IVUS image that clearly displays key structural parameters of the lesion, including its location, length and degree of stenosis, in addition to confirming proper stent placement. At the same time, the system performs spectroscopic analysis of optical data to produce a chemogram map that indicates the location of lipid core plaques and quantifies their lipid core burden. Integrating and co-registering the chemogram with IVUS provides immediate and valuable information to interventional cardiologists during the cardiac catheterization procedure.
For more information: www.infraredx.com


 November 14, 2025
November 14, 2025 









