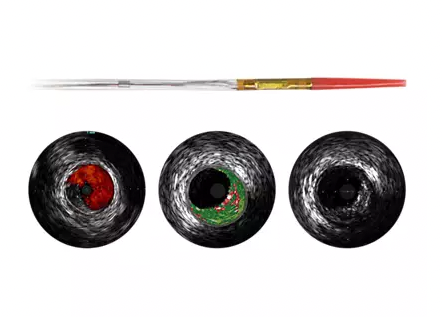
May 31, 2022 — Innovative Health, LLC, a specialty cardiology reprocessor, announced that the company has received clearance to reprocess the Philips Eagle Eye Platinum digital IVUS (intravascular ultrasound) catheter. This clearance effectively marks Innovative Health’s entry into the cath lab space, a move that will dramatically increase the potential costing savings of hospitals through reprocessing.
Primarily used in cath labs for interventional cardiology procedures, the Eagle Eye represents Innovative Health’s first non-electrophysiology catheter reprocessing clearance. The company has spent the past seven years building a full portfolio of FDA reprocessing clearances in the EP lab, producing substantial savings and allowing EP labs and hospitals to free up resources to improve patient care.
“Cardiac cath labs typically have no experience when it comes to reprocessing medical devices, but the savings potential is significant—and sorely needed,” said Innovative Health CEO Rick Ferreira. “We are excited to extend Innovative Health’s success in the EP lab to the cath lab, where we are confident we can help reduce procedure costs so hospitals can invest more in patient care and new technology. We’ve spent a considerable amount of time talking to service line managers and physicians in the cath lab, and they’re excited about the new opportunities that reprocessing can open for their practices.”
By using reprocessed Eagle Eye catheters, which are employed across many cath lab procedures, cath labs stand to save hundreds of dollars per procedure. At Innovative Health, the Eagle Eye is just the beginning. The company is currently preparing to build a substantial portfolio of clearances in the cath lab to deliver increased savings.
Prior to branching its reprocessing services into the cath lab, Innovative Health spent considerable time and resources getting to know the needs and challenges of the space. This research period culminated in a January 2022 roundtable of cath lab clinicians, technologists, administrators, and consultants. During this roundtable, Innovative Health explored the attractiveness and opportunity of reprocessing in the cath lab, which products would prove most relevant for reprocessing, what barriers reprocessing might encounter in the cath lab, and what factors could enable reprocessing to become a short-term success for the cath lab. Innovative Health has used these insights to establish its initial cath lab roadmap and plans to continually check in with the medical and hospital administrative community to refine and expand its services and offerings.
For more information: https://innovative-health.com/


 November 14, 2025
November 14, 2025 









