July 12, 2010 – Lantheus Medical Imaging Inc. today announced that it has acquired the balance of the worldwide rights for gadofosveset trisodium, a unique, injectable magnetic resonance angiography (MRA) blood pool imaging agent that it currently markets in the United States as Ablavar. Ablavar is indicated for use in adults with known or suspected peripheral vascular disease to evaluate aortoiliac occlusive disease. Lantheus purchased the worldwide rights in a recent auction of the remaining assets of Epix Pharmaceuticals. The company already owns exclusive rights to Ablavar in the United States, Canada and Australia.
“Obtaining the balance of the worldwide ownership of Ablavar is part of our strategy to broaden our portfolio of medically important diagnostic imaging products,” said Don Kiepert, President and Chief Executive Officer of Lantheus Medical Imaging Inc. “Our goal is to make this novel blood pool imaging agent available to patients and physicians on an expanded basis.”
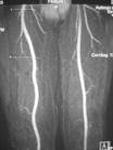
Ablavar is the first and only blood pool contrast agent approved for magnetic resonance angiography to evaluate aortoiliac occlusive disease (AIOD) in patients with known or suspected peripheral vascular disease. The albumin-binding properties of Ablavar make it designed for vascular imaging, allowing multiple images to be obtained using a single, low dose injection. Ablavar is clinically proven to produce high resolution MRA images, combining both dynamic (first pass) and steady state imaging, resulting in diagnostic accuracy comparable to conventional X-ray angiography, the current standard of care for diagnosing vascular disease such as AIOD.
The compound was formerly marketed as Vasovist outside the United States. Lantheus acquired exclusive rights for Ablavar in the United States, Canada and Australia in April 2009, and the product was launched in the United States in January 2010.


 August 17, 2023
August 17, 2023 








