November 13, 2013 — Lantheus Medical Imaging, Inc., a developer, manufacturer and distributor of diagnostic
imaging agents, announced preliminary results from the first of two planned Phase 3
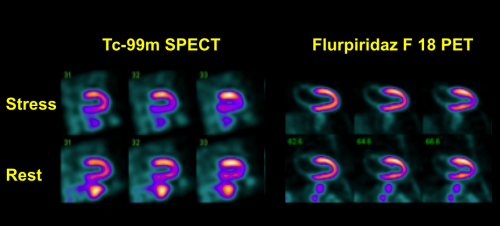
trials to assess the diagnostic efficacy of flurpiridaz F 18, an imaging agent used in positron emission tomography (PET) myocardial perfusion imaging (MPI) for the detection of coronary artery disease (CAD). The trial compared PET MPI with flurpiridaz F 18 to single photon emission computed tomography (SPECT) MPI using invasive coronary angiography as the truth standard. The open-label, multicenter study had two co-primary endpoints: superiority in sensitivity and non-inferiority in specificity. Flurpiridaz F 18 outperformed SPECT in a highly statistically significant manner in sensitivity and showed a statistical trend towards improved diagnostic accuracy. However, flurpiridaz F 18 did not meet the non-inferiority criterion for identifying subjects without disease.
“While preliminary results of this trial show that flurpiridaz F 18 missed one of the two co-primary endpoints, when looking at key secondary endpoints, such as image quality and diagnostic certainty, the image quality seen with flurpiridaz F 18 in the study is impressive, both absolutely and in comparison to SPECT, leading to a statistically significant improvement in diagnostic certainty,” said Cesare Orlandi, M.D., FACC, chief medical officer, Lantheus. “Image quality is very important in nuclear cardiology, since it allows diagnosing presence or absence of disease with greater confidence, which may lead to a decreased need for patient retesting.”
“We remain committed to our flurpiridaz F 18 clinical program,” said Jeff Bailey, president and CEO, Lantheus. “Next steps will include further image and data analysis, and meeting with our clinical advisors and the FDA. We will explore potential modifications to our clinical development plan and determine how we can use the results of this trial to advance the Phase 3 program for this exciting diagnostic candidate.”
“I continue to believe this novel agent is likely to represent the next advance in nuclear cardiology,” said Jamshid Maddahi, M.D., FACC, FASNC, professor of molecular and medical pharmacology (nuclear Medicine) and medicine (cardiology), David Geffen School of Medicine, UCLA and lead investigator. “Flurpiridaz F 18 has consistently demonstrated an ability to provide higher quality images and superior diagnostic performance than the current standard of care. In this study, the greater PET image resolution may have contributed to missing the non-inferiority specificity endpoint. More importantly, the SPECT results showed surprisingly low sensitivity and elevated specificity, which is inconsistent with prior studies, including the flurpiridaz F 18 Phase 2 trial. These findings may have confounded the outcome of the trial. From a safety perspective, the agent appears to be well-tolerated and the prospect of unit dose availability would make this candidate an attractive alternative to currently available diagnostic agents.”
For more information: www.lantheus.com