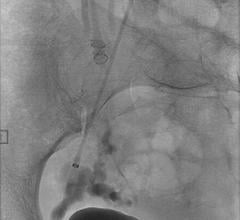
October 28, 2008 - The Los Angeles Brain and Spine Institute yesterday said George Rappard, M.D., a neurointerventional surgeon in Glendale, CA is the first physician west of the Rockies to treat a patient using an FDA-approved liquid system for treating wide-necked brain aneurysms.
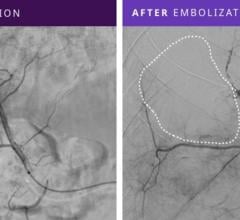
Dr. Rappard is one of only a few physicians nationwide that are presently exploring the use of a liquid embolic system to fill wide-necked brain aneurysms, which have a wide opening where the aneurysm arises from the artery or blood vessel. A brain aneurysm is a weakness in a major blood vessel that causes a portion of the vessel wall to balloon out. This abnormality puts an individual at risk should the aneurysm break open and bleed.
“A wide-necked brain aneurysm occurs in about 25 percent of patients with brain aneurysms,” said Dr. Rappard. “Wide-necked aneurysms can be difficult to treat surgically, which requires removal of bone and manipulation of the brain. However, by using a new liquid treatment called Onyx HD 500, we are able to use a minimally invasive endovascular procedure to treat the aneurysm from within the blood vessel.”
To date, there have been no research studies conducted to show whether this new liquid system is effective for treating wide-neck aneurysms, but initial clinical results are encouraging.
“The potential benefit of the liquid embolic system may be the complete blockage of the blood supply to the aneurysm,” Dr. Rappard said. “This would make a recurrence of the aneurysm less likely than seen with current treatment methods. It may also help to correct or lessen some symptoms.”
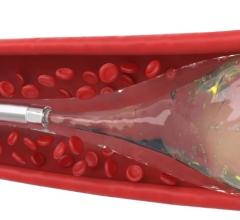
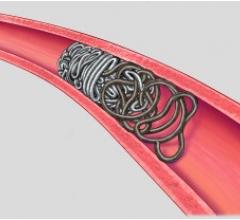
Until now, the only minimally invasive option available for treating brain aneurysms was coiling. In the coiling procedure, the surgeon will navigate a small catheter from the groin to the brain aneurysm and fill the aneurysm with metallic coils, causing clotting of the aneurysm. However, there is the possibility that the clot may dissolve, resulting in a recurrence of the aneurysm. In addition, some wide-necked aneurysms have such a large opening that the coils may not stay inside the aneurysm sac. In contrast to coiling, by filling the aneurysm sac or pocket with the Onyx liquid, which solidifies in minutes, blood flow into the aneurysm is blocked, helping to prevent the aneurysm from rupturing or increasing in size.
The release of the liquid treatment was preceded by a lengthy period of education and training for a small group of leading U.S. neurovascular specialists. Glendale Adventist Medical Center is one of about 25 hospitals in the U.S. to perform this procedure as an alternative to conventional surgery.
The new Onyx liquid treatment made by ev3 has been FDA approved under a humanitarian device exemption, which allows physicians to use the liquid to treat a disease or condition that affects fewer than 4,000 individuals in the U.S. per year and for which no comparable device is available. To qualify for treatment, patients must possess intracranial, saccular, sidewall aneurysms that present with a wide neck (4 mm) or with a dome-to-neck ratio
For more information: www.ev3.net, www.ninds.nih.gov, www.strokeassociation.org, www.snisonline.org


 June 05, 2025
June 05, 2025