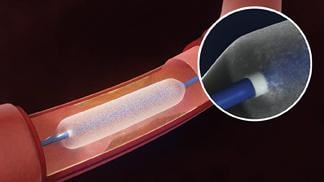
November 7, 2018 — The Lutonix Drug-Coated Balloon (DCB) showed statistically significant safety equivalence with a standard percutaneous transluminal angioplasty (PTA) catheter for the treatment of narrowed or obstructed arteries below the knee in recent trial results. The data were presented at the Vascular Interventional Advances (VIVA) 2018 annual conference, Nov. 5-8 in Las Vegas.
The Lutonix DCB investigational device exemption (IDE) clinical study is a prospective, global, multicenter, randomized, controlled trial comparing the Lutonix 014 DCB to standard angioplasty for the treatment of narrowed or obstructed arteries below the knee (BTK). The study used both proportional/binary and Kaplan Meier analyses to assess safety and efficacy. The primary safety endpoint — freedom from composite all-cause death, above the ankle or major reintervention of the treated limb through 30 days — was met showing statistically significant safety equivalence between the Lutonix 014 DCB and standard PTA catheter, in both the proportional/binary and Kaplan Meier analyses.
“The six-month clinical data from the Lutonix BTK trial represent the beginning of a paradigm shift in the treatment of patients with critical limb ischemia (CLI),” said Jihad Mustapha, M.D., FACC, FSCAI, Advanced Cardiac and Vascular Amputation Centers, Grand Rapids, Mich. “The initial results are extremely encouraging and give new hope to patients with CLI.”
The primary efficacy endpoint was assessed using a composite measurement of limbs saved from amputation and the openness of arteries, known as primary patency. By proportional/binary analysis, at six months there was an improvement in primary efficacy of 10.2 percent (DCB: 73.7 percent and PTA: 63.5 percent, p=0.0273, not-significant). The more commonly used Kaplan Meier analysis of the primary efficacy endpoint demonstrated a significant difference of 14.6 percent (DCB: 85.3 percent, PTA: 70.7 percent, p<0.001). Additional analyses are planned for 12, 24 and 36-month follow-ups.
The trial included approximately 450 participants of which 91 percent had CLI, a severe form of vascular disease that seriously decreases blood flow to the lower leg arteries.
An estimated 3.4 million people in the United States have CLI today, and the number is estimated to grow to nearly 3.8 million by 2020.[i] CLI is associated with a six-month major amputation rate ranging from 10 percent to 40 percent and with mortality rates as high as 50 percent at five years.[ii]
The Lutonix 014 DCB has been commercially available in Europe, Canada and Australia for treatment of the below-the-knee arteries associated with CLI since 2013.
For more information: www.vivaphysicians.org
References
[i]. Yost, Mary. United States Critical Limb Ischemia by Rutherford Category Prevalence and Markets in Patients and Limbs, The Sage Group. 2018.
[ii]. Teraa M, et al. Critical Limb Ischemia: Current Trends and Future Directions J Am Heart Assoc. 2016;5:e002938.


 January 05, 2026
January 05, 2026 









