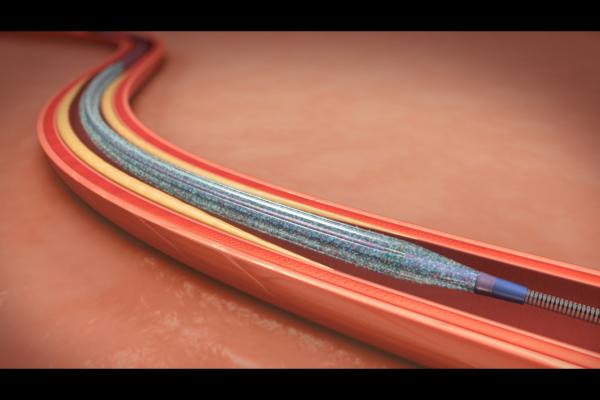
February 23, 2015 — Medtronic announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has approved a transitional pass-through payment for the company’s IN.PACT Admiral drug-coated balloon (DCB) under the Medicare hospital outpatient prospective payment system (OPPS). The decision removes a potential barrier to patient access to this new medical device, which represents an improvement to the standard of care for peripheral arterial disease in the upper leg.
This supplemental reimbursement provision takes effect on April 1, 2015, and will remain in effect for the following two to three years. It aims to cover the additional cost to U.S. hospitals for treating Medicare beneficiaries with the IN.PACT Admiral DCB in the outpatient setting. The Healthcare Common Procedure Coding System (HCPCS) code for this new device category will be C2623 (catheter, transluminal angioplasty, drug-coated, non-laser).
A similar supplemental payment to hospitals that treat Medicare beneficiaries with the device on an inpatient basis is currently under review by CMS. A decision on a new technology add-on payment for the hospital inpatient prospective payment system (IPPS) is expected over the summer.
The IN.PACT Admiral DCB is designed to reopen superficial femoral and popliteal arteries that have been narrowed or blocked by plaque. Once deployed in the artery, the balloon delivers a proven, safe and effective dose of the anti-restenotic drug paclitaxel to the artery walls. The drug aims to prevent the artery from narrowing again by minimizing scar tissue formation.
For more information: www.medtronic.com


 June 13, 2024
June 13, 2024 









