September 21, 2021 — Medtronic is recalling its Pipeline Flex Embolization Device and Pipeline Flex Embolization Device with Shield Technology, which are transcatheter devices used to seal brain aneurysms. The company said there is a risk of the delivery system’s wire and tubes fracturing and breaking off when the system is being used to place, retrieve, or move the stent inside a patient.
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Fractured pieces could be left inside the patient’s brain bloodstream. It is also possible that attempts to retrieve the fractured pieces may make the patient’s condition worse. The fragments can also cause other serious adverse health consequences such as continued blockage of blood vessels, stroke, and death.
There have been 59 reported device malfunctions, 10 serious injuries, and two deaths related to this recall.
The recalled includes 8,825 devices in the United States that were distribution between April 18, 2019 to Aug. 13, 2020.
Medtronic is the parent company of Micro Therapeutics Inc., ev3 Neurovascular, which makes the devices.
A recall letter was sent to all customers July 13, 2021, requesting them to:
• Stop use of any impacted product.
• All unused impacted products should be immediately quarantined.
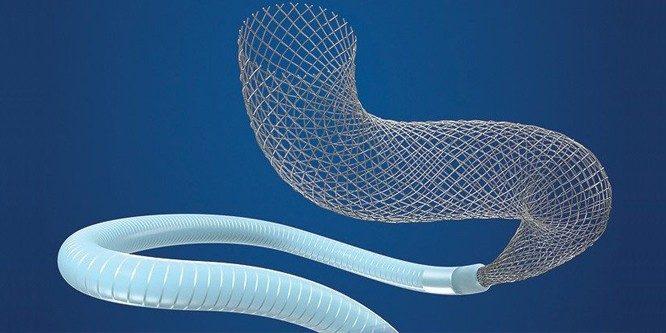
Design and Function of the Pipeline Flex Embolization System
The Pipeline Flex Embolization Device and Pipeline Flex Embolization Device with Shield Technology are permanent mesh stent braided from platinum and tungsten and cobalt-chromium-nickel alloy wires. These devices are intended for the treatment of brain aneurysms that bulge or balloon out the sides of the blood vessel (wide-neck and fusiform). The Pipeline Flex Devices include a guidewire-based delivery system used to place the implant inside the patient.
Recalled Pipeline Flex Products:
• Pipeline Flex Embolization Device and Pipeline Flex Embolization Device with Shield Technology.
• Pipeline Flex Embolization Device Models Numbers: PED-250-XX, PED-275-XX, PED-300-XX, PED-325-XX, PED-350-XX, PED-375-XX, PED-400-XX, PED-425-XX, PED-450-XX, PED-475-XX, PED-500-XX.
• Pipeline Flex Embolization Device with Shield Technology: PED2-250-XX, PED2-275-XX, PED2-300-XX, PED2-325-XX, PED2-350-XX, PED2-375-XX, PED2-400-XX, PED2-425-XX, PED2-450-XX, PED2-475-XX, PED2-500-XX
How to Return Recalled Products
The Vendor is asking for the return of all impacted products to Medtronic. Facilities can contact their Medtronic representative for help with product returns or to identify a suitable replacement product if one is needed.
Complete and return the customer response form via fax to Medtronic at 1-651- 367-7075, Attention: Neurovascular Quality or email it to [email protected].
Medtronic Contact Information
Customers with questions about this recall can contact the recalling Medtronic’s Quality Assurance via email at [email protected] or by calling 1(800) 633-8766 (U.S. toll free).
Additional Information on the Pipeline Flex Recall:
• FDA Recall announcement
• Pipeline Flex Medical Device Recall Database Entry
• Pipeline Flex with Shield Technology Medical Device Recall Database Entry
To report an issue with these devices, people can use the FDA adverse event reporting system.


 November 14, 2025
November 14, 2025 









