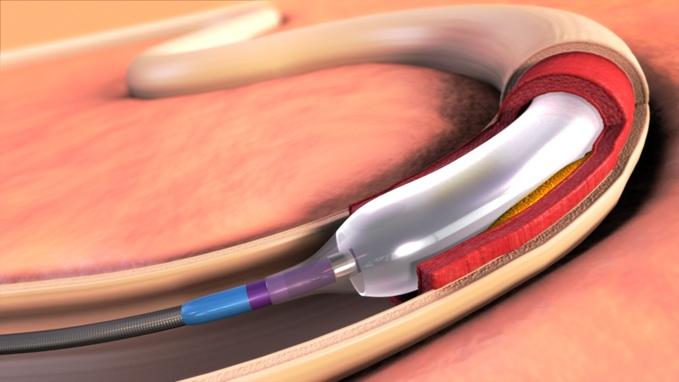
February 6, 2015 — United States hospitals began using a new medical device from Medtronic plc called the In.Pact Admiral drug-coated balloon (DCB) to treat patients with peripheral arterial disease (PAD) in the upper leg. PAD is a common cardiovascular condition that causes leg pain and increases the risk of heart attack and stroke.
Recently approved by the U.S. Food and Drug Administration (FDA), the In.Pact Admiral DCB offers patients a new therapy option that has been proven to reduce the need for costly repeat procedures that are commonly associated with other available interventional therapies.
The first uses of the new medical device following FDA approval took place at NewYork-Presbyterian Hospital/Columbia University Medical Center by William Gray, M.D.; Detroit Medical Center’s Harper Hospital in Michigan by Mahir Elder, M.D.; Yuma Regional Medical Center in Arizona by Joseph Cardenas, M.D., of the Heart Center of Yuma; and Terrebonne General Medical Center in Houma, Louisiana by Craig Walker, M.D., of Cardiovascular Institute of the South.
“As an investigator in the clinical trial that contributed to this device’s FDA approval, I have seen firsthand how well the In.Pact Admiral drug-coated balloon works as a treatment for peripheral arterial disease in the upper leg,” said Gray. “Based on the trial results, which were recently published in the journal Circulation, I see the In.Pact Admiral DCB fast becoming a first-line therapy option for patients with this condition.”
The United States launch of the device begins about a week after Medtronic completed its acquisition of Covidien. This acquisition significantly expands Medtronic’s peripheral vascular sales force, which will facilitate access to the new device.
The device is designed to reopen arteries located in the upper leg, specifically the superficial femoral and popliteal arteries, when they have been narrowed or blocked by plaque. Once deployed in the artery, the balloon delivers a proven, safe and effective dose of the anti-restenotic drug paclitaxel to the artery walls. The drug aims to prevent the artery from narrowing again by minimizing scar tissue formation.
The DCB arm of the In.Pact SFA Trial demonstrated the lowest clinically-driven target lesion revascularization (CD-TLR) rate ever reported for an interventional treatment of PAD in the superficial femoral artery (SFA), with only 2.4 percent of patients treated with the device requiring a repeat procedure at one year, compared to one in five patients (20.6 percent) treated with percutaneous transluminal angioplasty (PTA).
The data also revealed the highest reported rates of primary patency, which measures sustained restoration of adequate blood flow through the treated segment of the artery. Based on Kaplan-Meier survival estimates for primary patency at 360 days, the data showed an 89.8 percent sustained restoration of blood flow in the DCB group compared to 66.8 percent for the PTA group. Using the trial’s protocol definition, primary patency assessed at 12 months of follow up was 82.2 percent for the DCB group and 52.4 percent for the PTA group.
Results from an interim economic analysis of the In.Pact SFA Trial revealed that treatment with the DCB is cost-effective compared to balloon angioplasty from discharge through one-year of follow-up, indicating the potential to lower overall healthcare costs over the longer term.
For more information: www.medtronic.com


 June 13, 2024
June 13, 2024 









