Image credit: Getty Images
March 8, 2022 – MemorialCare Heart & Vascular Institute (MHVI), through its advanced research program, is participating in a CSL Behring-sponsored multi-center, Phase III clinical trial – "ApoA-I Event reducinG in Ischemic Syndromes II (AEGIS-II) – which is studying the removal of cholesterol from the arteries to reduce the risk of a second heart attack or stroke in patients. All three adult hospitals within MemorialCare's health system — MemorialCare Long Beach Medical Center, MemorialCare Orange Coast Medical Center in Fountain Valley, and MemorialCare Saddleback Medical Center in Laguna Hills — are providing access to this study to their cardiac patients.
According to the World Health Organization (WHO), cardiovascular (CV) disease is the leading cause of death globally, with an estimated 800,000 heart attacks occurring each year in the U.S. alone. Of these, 600,000 are a first heart attack and 200,000 happen to people who have already suffered a heart attack, according to the Centers for Disease Control and Prevention (CDC).
"While we have made tremendous strides in preventing adverse cardiac events, this study further enables us to explore the real possibility of cardiologists being able to potentially provide a viable therapy for patients who have suffered a heart attack and are thus at risk for potentially suffering another one," says John Bahadorani, M.D., interventional cardiologist, MemorialCare Saddleback Medical Center.
The study will evaluate the efficacy and safety of CSL112 in reducing the risk of major adverse cardiovascular events (MACE) in patients with acute coronary syndrome (ACS) diagnosed with either ST-segment elevation myocardial infarction (STEMI) or non-ST-segment elevation myocardial infarction (NSTEMI), including those managed with percutaneous coronary intervention (PCI) or medically managed. MACE is defined as heart attack, stroke or cardiovascular death.
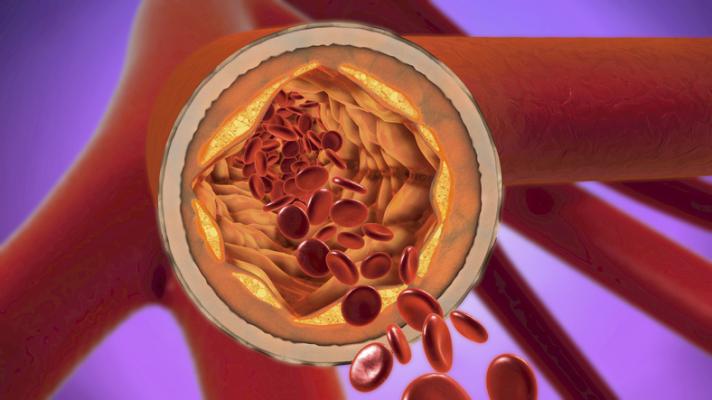
"Apolipoprotein A-I is the protein backbone of high-density cholesterol (HDL), known as the 'good cholesterol.' The novel medication, CSL112, is a synthetic form of Apolipoprotein A-I, which when infused into the bloodstream bumps up HDL resulting in cholesterol actually being removed from blockages in the arteries," describes Todd Zynda, D.O., interventional cardiologist at MemorialCare Long Beach Medical Center.
"This infusion therapy is a game changer because with this study we are able to administer this potentially lifesaving intravenous treatment directly into the bloodstream soon after the onset of a cardiac event, allowing us the potential to clear the blockages that cause heart attack," says Sarah Elsayed, M.D., interventional cardiologist, MemorialCare Orange Coast Medical Center.
Since the onset of the pandemic, many people have delayed receiving important clinical evaluation and preventative care to help effectively manage high blood pressure and/or cholesterol. As a result, an increasing number of patients are being treated for cardiovascular conditions, such as stroke and heart attack. Therefore, it's crucial that hospitals possessing extensive clinical expertise and a dedicated research team, make these types of advanced research studies available to their patients.
"The MemorialCare Heart & Vascular Institute is proud to continue MemorialCare's tireless efforts to remain at the forefront of innovative clinical research studies," says David Shavelle, M.D., medical director, adult cardiology and interventional laboratory, MemorialCare Long Beach Medical Center. "These studies can have a very positive impact on the health of the communities we serve, throughout Los Angeles to Orange counties."
For more information: https://www.memorialcare.org


 January 05, 2026
January 05, 2026 









