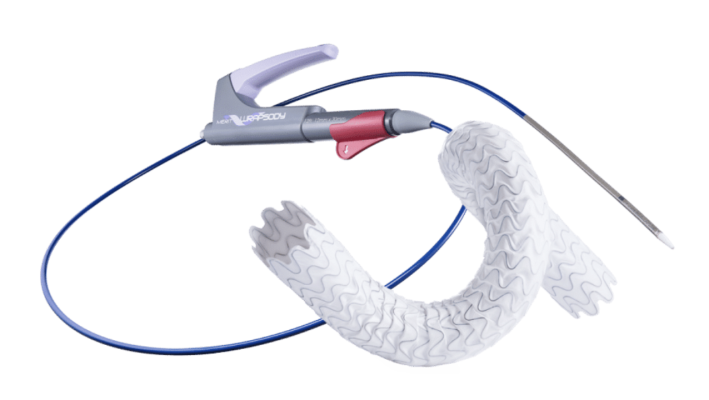
August 3, 2023 — Merit Medical Systems, a leading global manufacturer and marketer of healthcare technology, today announced the completion of enrollment in its WRAPSODY Arteriovenous Access Efficacy(WAVE) pivotal study. Merit’s WAVE study is a prospective, randomized, controlled, multicenter study comparing the Merit WRAPSODY Cell-Impermeable Endoprosthesis(CIE) to percutaneous transluminal angioplasty for treatment of stenosis/occlusion in the venous outflow circuit in patients undergoing hemodialysis.
Creation and maintenance of an arteriovenous fistula or graft (AVF/AVG) to achieve long-term vascular access (access to blood vessels) is required for patients undergoing hemodialysis. However, progressive stenosis (narrowing) and/or occlusion (blockage) of blood vessels where the AVF and AVG are located can prevent delivery of hemodialysis, which can have life-threatening consequences. WRAPSODY was developed to help physicians treat patients with stenosis/occlusion in the vessels used for hemodialysis.
The WAVE study enrolled 244 patients with AVFs and 113 patients with AVGs across sites in Brazil, Canada, the United Kingdom, and the United States. Merit intends to collect safety and efficacy outcomes throughout the study follow-up period. Merit anticipates filing primary outcomes with the U.S. Food and Drug Administration (FDA) for Premarket Approval (PMA) after six months post-enrollment completion. Merit intends to follow patients enrolled in the WAVE study for 24 months following completion of enrollment.
“Given the inadequacy of therapeutic options to maintain vascular access in hemodialysis patients, understanding WRAPSODY’s overall performance—due to its unique cell-impermeable stent covering in helping to maintain AV fistulas and grafts—is of high value to physicians and the scientific community,” says Mahmood K. Razavi, MD, FSIR, FSVM, medical director of clinical research at St. Joseph Heart and Vascular Center in Orange, Calif., co-principal investigator of the WAVE study, and paid consultant of Merit.
“The WAVE study has now completed the anticipated recruitment thanks to the patients that participated and to the hard work of all the global investigators and their respective teams,” says Rob Jones, MBChB, MRCP, FRCR, consultant interventional radiologist at Queen Elizabeth Hospital Birmingham in Birmingham, England, co-principal investigator of the WAVE study, and paid consultant of Merit. “I am looking forward to the data analysis and the next step in this device’s journey in providing definitive management to AV access patients.”
“Enrollment of the last patients in the WAVE study represents an important milestone,” says Fred P. Lampropoulos, Merit’s Chairman and CEO. “We believe it will enable us to provide critical insight to our physician partners and support our submission of a PMA Application to the Food and Drug Administration.”
The Merit WRAPSODY Cell-Impermeable Endoprosthesis is not approved, cleared, or available for commercial distribution in the United States and may not be approved, cleared, or available for sale or use in other countries. In the United States, the device is being used under an Investigational Device Exemption (IDE) from the Food and Drug Administration. Findings from the WAVE study are expected to expand on results from the first-in-human study (WRAPSODY FIRST) and support the Premarket Approval (PMA) application to the FDA for commercial use in the United States. The WRAPSODY device previously received the Conformité Européenne (CE) Mark for commercial use in the European Union and is available in Brazil.
For more information: https://www.merit.com/


 January 15, 2026
January 15, 2026 









