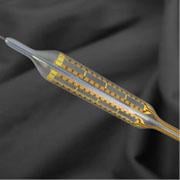
Lateral view of the Minnow Medical RF catheter for treating PAD.
April 16, 2009 - Minnow Medical this week received the CE mark, clearing its disposable Guided Reshaping Technology (GRT) catheter to treat peripheral artery disease (PAD) for marketing in the European Union.
The Minnow’s GRT treats artery disease by precise delivery of controlled radio frequency (RF) energy to open, diseased arteries. The company said RF energy is delivered through electrodes on the surface of a specially-designed disposable angioplasty balloon catheter. The company believes its technology will help reduce the use of stents in peripheral procedures.
Minnow is currently conducting clinical trials in Europe with a first-generation version of its catheter system.
PAD affects approximately 27 million people in North America and Europe, and many of the current treatments provide only limited or short-term benefit. The PAD device treatment market is estimated at $1.2 billion per year in the U.S. alone.
For more information: www.minnowmedical.com


 October 28, 2025
October 28, 2025 









