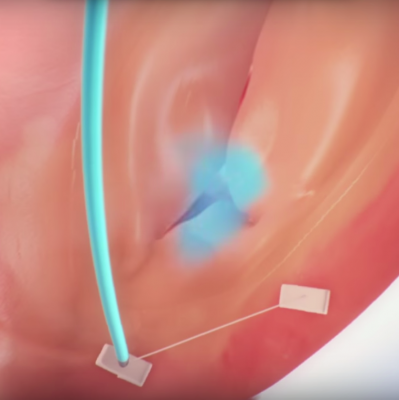
December 10, 2015 — Mitralign Inc. announced the first U.S. subject has been enrolled in the SCOUT Study using the company’s Trialign (percutaneous tricuspid valve annuloplasty) System. The procedure was performed by Rebecca Hahn, M.D., director of interventional echocardiography, principal investigator for the SCOUT study, and Susheel Kodali, M.D., interventional cardiologist and director, Structural Heart and Valve Program at NewYork-Presbyterian/Columbia University Medical Center.
“We are extremely excited to be pioneering a novel solution in percutaneous repair for the tricuspid valve,” commented Hahn. “Given the reports that operative mortality for tricuspid valve replacement (TVR) surgery can top 30 percent,coupled with the lack of treatment options, this system represents a very welcome advancement.”
SCOUT is a United States-based early feasibility Investigational Device Exemption study using the Trialign system in subjects with symptomatic chronic functional tricuspid regurgitation (FTR). It will assess the early safety and feasibility of the device for the treatment of tricuspid regurgitation in subjects with a minimum of moderate tricuspid regurgitation and in whom left‐sided valve surgery is not planned.
Tricuspid regurgitation, sometimes called tricuspid insufficiency, occurs when the tricuspid valve fails to open and close properly, causing blood to flow backwards into the right atrium. It is estimated that 50 percent of patients with mitral regurgitation have moderate to severe tricuspid regurgitation. However, in the United States, surgeons treat only 5,500 patients per year, most of them in conjunction with left heart surgeries. When treating the valves, surgeons choose repair 90 percent of the time versus replacement 10 percent. If left untreated, TR can weaken the heart, leading to heart enlargement and ultimately progressing to heart failure.
For more information: www.mitralign.com


 January 05, 2026
January 05, 2026 









