January 18, 2012 — PLC Systems Inc. announced that final results from the MYTHOS investigator-sponsored clinical trial of RenalGuard in Italy have been published in the January 2012 issue of Journal of the American College of Cardiology – Cardiovascular Interventions, a peer-reviewed journal of the American College of Cardiology. The results from this trial showed RenalGuard as better than the current standard-of-care at reducing rates of contrast-induced nephropathy (CIN) and in-hospital dialysis in at-risk patients undergoing certain imaging procedures.
Initial results from this study were first presented at Transcatheter Cardiovascular Therapeutics (TCT) in September 2010.
Mark R. Tauscher, president and CEO of PLC Systems, said, "The positive results of the MYTHOS clinical trial demonstrate, in scientifically significant findings, that RenalGuard is a superior approach to reducing the incidence of CIN in at-risk patients."
The trial enrolled 170 patients with chronic kidney disease (CKD) who underwent elective or urgent percutaneous coronary interventions (PCI) in Italy. The results indicate that patients who were at higher risk for renal failure and were treated with RenalGuard while undergoing imaging procedures developed CIN at 74 percent lower rate than those treated with overnight hydration. The trial also found that patients treated with RenalGuard had significantly fewer in-hospital adverse events.
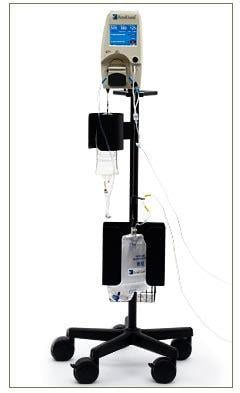
The RenalGuard system is comprised of a console and a single use set for infusion and urine collection. It connects to a patient's Foley catheter, and an infusion connects to a standard IV catheter. The console measures the volume of urine in the collection set and infuses an equal volume of hydration fluid to match the patient's urine output.
For more information: www.sciencedirect.com, www.plcmed.com


 January 05, 2026
January 05, 2026 









