November 21, 2009 – The first randomized intracardiac arrest cooling study performed using a intra-nasal cooling method showed promising results. Findings included much faster and earlier cooling in treated patients and significantly higher neurologically intact survival to discharge rate in many patients.
The Pre-Resuscitation Intra-Nasal Cooling Effectiveness (PRINCE) study involved 200 patients and was conducted by 15 Emergency Medical Systems (EMS) in Belgium, Germany, Italy, Czech Republic and Sweden. The aim was to determine safety and efficacy of intra-nasal cooling during ongoing resuscitation of cardiac arrest patients even before the return of circulation (ROSC).
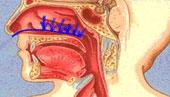
The study was conducted using RhinoChill, a non-invasive nasal catheter that sprays a rapidly evaporating coolant liquid into the nasal cavity. This large cavity is a heat exchanger and lies right under the brain.
The trial was designed to determine the safety and effectiveness of early cooling initiated at the site of the arrest. The RhinoChill technology enabled cooling to start much earlier than is possible with conventional methods used in a hospital setting and focuses on the brain.
“The brain is the organ that dies first so the closer to the time of arrest the brain is cooled, the more of it is rescued,” said Denise Barbut, M.D, founder and CEO of BeneChill, the company that makes RhinoChill. “The brain is the organ that controls the heart, much like a puppet on a string,” she added.
Additional endpoints included cooling rates, time to achieve target temperature, ease of use in the field, ROSC rates, survival and neurologically intact survival. EMS personnel recruited adults over 18 years old who were in cardiac arrest and not hospitalized during resuscitation. All patients who were deemed eligible for advance cardiac life support (ACLS) were included as long as the arrest was witnessed and cardiopulmonary resuscitation (CPR) was initiated within 20 minutes of collapse.
The results of the study included:
• Cooling was initiated 23 minutes following arrest and lowered brain
temperature (tympanic) (34.2 degrees C vs. 35.5 degrees C) and body
(core) temperature (35.1 degrees C vs. 35.8 degrees C) significantly
by ER arrival.
• Time to target tympanic temperature of 34 degrees was three hours
faster and time to target core temperature was two hours faster in
patients cooled intra-nasally in the field compared to those receiving
hospital cooling alone.
• Survival to discharge was higher in treated patients admitted to hospital (46.7 vs 31 percent) and significantly higher in those in whom CPR was initiated within 10 minutes of collapse, irrespective of rhythm
(59.1 vs. 29.4 percent).
• Neurologically intact survival to discharge was higher in treated patients admitted to the hospital (36.7 vs. 21.4 percent) and significantly
higher in those in whom CPR was initiated within 10 minutes of collapse, irrespective of rhythm (45.5 vs. 17.6 percent).
• Intranasal cooling with RhinoChill was feasible and safe during an arrest. Nasal discoloration was the most commonly reported adverse
event occurring in 13 patients. This resolved spontaneously in all patients who were successfully resuscitated.
“In this study, early cooling of the brain combined with early CPR favorably affected outcomes, irrespective of rhythm,” said Maaret Castren, M.D, Ph.D. of the department of clinical science and education, Karolinska Institute, Stockholm, Sweden and the department of emergency medicine, Sodersjukhuset and PRINCE lead investigator.
“We believe that this study demonstrates that making every attempt to initiate both CPR and intra-arrest cooling as early as possible in the resuscitation process should be adopted,” Dr. Castren added.
The EMS teams also noted that the portability of the device and ease of use meant that cooling could be administered in the field by non-specialized medical personnel. This also is useful in the hospital setting where the patient can be transported around with on-going cooling.
Dr. Castren presented the findings in Orlando, Fla., on Nov. 15 during the American Heart Association’s (AHA) Resuscitation Science Symposium “Best of the Best” presentations.
RhinoChill will be marketed in Europe in early 2010, but is not available in the United States.
For more information: www.benechill.com


 January 05, 2026
January 05, 2026 









