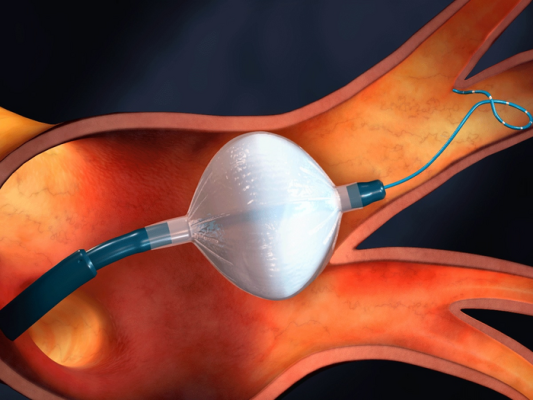
Cryoablation involves guiding a small tube into the heart and inflating a small balloon to kill problematic tissue with cold temperatures. Image courtesy of Medtronic
November 10, 2022 — A national study led by UBC researchers at the Centre for Cardiovascular Innovation is shedding light on how to more effectively treat atrial fibrillation (AF) – a common heart rhythm problem associated with increased risk of stroke and heart failure.
The study, published today in The New England Journal of Medicine, shows that early intervention with cryoballoon catheter ablation (cryoablation) is more effective at reducing the risk of serious long-term health impacts, when compared to the current first step in treatment, antiarrhythmic drugs.
“By treating patients with cryoablation right from the start, we see fewer people advancing to persistent, more life-threatening forms of atrial fibrillation,” says Dr. Jason Andrade, an associate professor of medicine at UBC and director of Heart Rhythm Services at Vancouver General Hospital. “In the short term, this can mean less recurrences of arrhythmia, improved quality of life and fewer visits to the hospital. In the long run, this can translate into a reduced risk of stroke and other serious heart problems.”
Cryoablation is a minimally invasive procedure that involves guiding a small tube into the heart to kill problematic tissue with cold temperatures. Historically, the procedure has been reserved as a secondary treatment when patients don’t respond to antiarrhythmic drugs.
“This study adds to the growing body of evidence that early intervention with cryoablation may be a more effective initial therapy in the appropriate patients,” says Dr. Andrade.
Early Intervention Halts Disease Progression
AF affects approximately three per cent of the population, or more than one million Canadians.
While the condition starts as an isolated electrical disorder, each recurring incident can cause electrical and structural changes in the heart that can lead to longer-lasting events known as persistent AF (episodes lasting more than seven continuous days).
“Atrial fibrillation is like a snowball rolling down a hill. With each atrial fibrillation episode there are progressive changes in the heart, and the heart rhythm problem gets worse,” explains Dr. Andrade.
The new findings, stemming from a multi-site clinical trial, show that cryoablation can stop this snowball effect.
For the trial, the pan-Canadian research team enrolled 303 patients with AF at 18 sites across Canada. Half of the patients were randomly selected to receive antiarrhythmic drugs, while the other half were treated with cryoablation. All patients received an implantable monitoring device that recorded their cardiac activity throughout the study period.
After three years, the researchers found that patients in the cryoablation group were less likely to progress to persistent AF compared to patients treated with antiarrhythmic drugs. Over the follow-up period, the cryoablation patients also had lower rates of hospitalization and experienced fewer serious adverse health events that resulted in death, functional disability or prolonged hospitalization.
Addressing the Root Cause
Because cryoablation targets and destroys the cells that initiate and perpetuate AF, the researchers say it can lead to longer-lasting benefits.
“With cryoablation, we’re treating the cause of the condition, instead of using medications to cover-up the symptoms,” says Dr. Andrade. “If we start with cryoablation, we may be able to fix atrial fibrillation early in its course.”
The new study builds on a previous paper in which Dr. Andrade and his team demonstrated that cryoablation was more effective than antiarrhythmic drugs at reducing the short-term recurrence of atrial fibrillation.
The researchers say that more effective early interventions would benefit patients as well as the health care system. Currently, costs associated with the provision of atrial fibrillation-associated care are estimated at 2.5 per cent of overall annual health care expenditures. Those costs are expected to rise to four per cent within the next two decades.
“The evidence shows increasingly that it’s time to rethink how we approach the treatment of atrial fibrillation. With effective early intervention, we can keep people healthy, happy, and out of hospital, which would be a tremendous benefit for patients and their families, and also our entire health system.”
For more information: https://www.ubc.ca/


 June 13, 2024
June 13, 2024 









