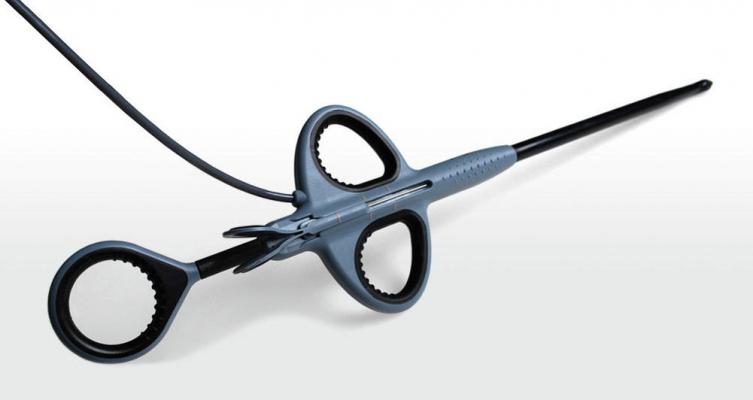
April 12, 2013 — NeoChord has received CE mark for its technology that allows the implantation of artificial chordae tendinae, a proven technique for the repair of mitral valve prolapse, via a transapical, off-pump procedure.
The company also announced that it has started the 50-patient Transapical Artificial Chordae Tendinae (TACT) Registry.
“Receiving CE marking for the NeoChord device for implantation of artificial chordae tendinae via a transapical, off-pump procedure and beginning the Tact Registry are meaningful steps forward in the treatment of patients with severe mitral regurgitation,” said Giovanni Speziali, M.D., the primary inventor of NeoChord’s technology. “We are pleased with the short- and medium-term clinical outcomes to date using NeoChord’s technology to treat patients suffering from mitral regurgitation. The NeoChord procedure allows patients an alternative to still undergo a quality repair without enduring the rigors of traditional treatment via open-chest surgery performed on a stopped heart.”
For more information: www.NeoChord.com


 January 05, 2026
January 05, 2026 









