
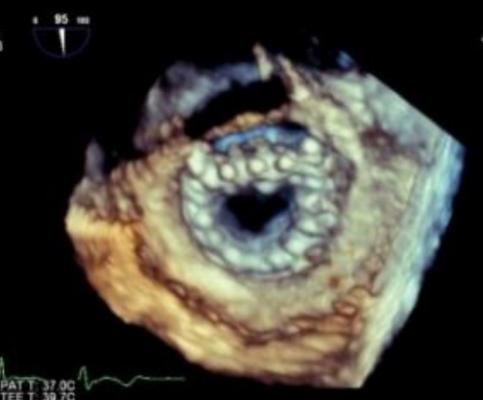
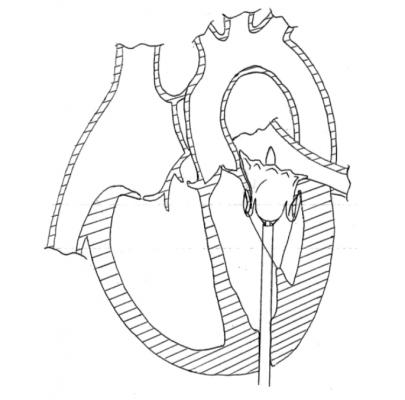
The Tiara transcatheter mitral valve.
September 18, 2012 — Neovasc Inc. announced that acute results from preclinical studies of its Tiara valve for the transcatheter treatment of mitral regurgitation were published in the Journal of the American College of Cardiology (JACC). The study reports that the initial experience with the Tiara transcatheter mitral valve was encouraging, and that implantation of Tiara valves was feasible, relatively straightforward and resulted in a securely-implanted, well-functioning device that maintained good hemodynamics in the test animals. The study, which will be published in the Oct. 9, 2012, edition of JACC, is currently available online.
“We are very pleased with the results of our Tiara implantations in preclinical animal models,” noted Alexei Marko, CEO of Neovasc. “Publication of the first results from the acute phase of these studies in a prestigious journal such as JACC highlights the potential value of Tiara for the treatment of patients with mitral regurgitation who cannot be treated surgically. We look forward to sharing long-term Tiara results from studies in chronic animal models at TCT 2012 next month.”
In the published study, Tiara valves were implanted successfully in 81 percent of the test animals, with total procedure times ranging from 17 to 26 minutes. In the successful implantations, angiographic and echo imaging demonstrated excellent function of the Tiara, with no obstruction of the left ventricular outflow tract, no pericardial effusion, no encroachment on the aortic valve, no transvalvular gradients and most importantly, no significant paravalvular leak. All animals remained hemodynamically stable during the implant procedure without the need for rapid pacing.
The report, "Tiara: A Novel Catheter-Based Mitral Valve Bioprosthesis: Initial Experiments and Short-Term Pre-Clinical Results," was authored by Shmuel Banai, M.D., E. Marc Jolicoeur, M.D., Marc Schwartz , RCIS, Patrick Garceau, M.D., Simon Biner, M.D., Jean-Francois Tanguay, M.D., Raymond Cartier, M.D., Stefan Verheye, M.D., Christopher J. White, M.D. and Elazer Edelman, M.D., Ph.D. It is being published in the Journal of the American College of Cardiology, Vol. 60, No. 15, 2012
For more information: content.onlinejacc.org/article.aspx?articleid=1358177


 January 05, 2026
January 05, 2026 









