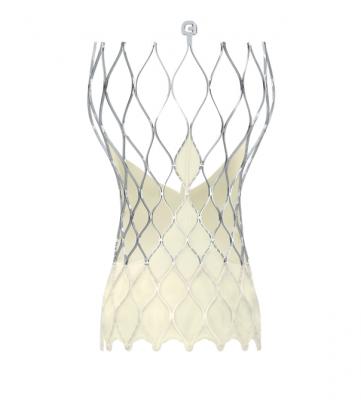
November 8, 2017 — Medtronic plc recently presented new clinical research to support the positive clinical performance of the Evolut Transcatheter Aortic Valve Replacement (TAVR) Platform in intermediate-risk, severe, symptomatic, aortic stenosis patients. Outcomes from the Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) Trial and the Evolut R FORWARD “real-world” study were presented at the 2017 Transcatheter Cardiovascular Therapeutics (TCT) Annual Meeting, Oct. 29-Nov. 2, in Denver, reinforcing the valve’s strong performance in healthier, more active patients.
Complete one-year outcomes from the SURTAVI trial based on Kaplan-Meier analyses confirmed the strong performance of the CoreValve and Evolut R TAVR systems versus the gold standard of open-heart surgery in an intermediate-risk severe symptomatic aortic stenosis patient population. The results indicate the platform continued to provide comparable results to surgery with regard to the primary combined endpoint of all-cause mortality or disabling stroke (7.8 percent for TAVR vs. 8.5 percent for surgery; p=0.55) at one year. A subgroup analysis of the SURTAVI Continued Access Study (CAS) showed consistently positive safety data for the Evolut R System in intermediate-risk patients with high rates of survival (100 percent) and low rates of all stroke (1.5 percent) or disabling stroke (0.4 percent) at 30-days.
“With its supra-annular and self-expanding design, the Evolut TAVR platform is well-suited to deliver excellent valve performance for intermediate-risk patients who are often considered to be more active than high- or extreme-risk patients,” said Nicolas M. Van Mieghem, M.D., director of interventional cardiology at Erasmus Medical Center in Rotterdam, the Netherlands, and presenter of the SURTAVI one-year data at the meeting. “As new clinical data are gathered for this patient population, we continue to see the exceptional clinical benefits this self-expanding valve provides as a minimally-invasive treatment alternative to surgery.”
Additionally, a sub-analysis from the SURTAVI trial where patients were stratified based on their STS Predicted Risk of Mortality (PROM) showed that TAVR patients demonstrated a significantly lower rate of all-cause mortality or disabling stroke at one year (1.5 percent vs. 6.5 percent; p=0.04) in the category of patients with STS-PROM < 3 percent. Patients treated with TAVR also showed mortality improvements across risk categories at one year, whereas surgical mortality rates were similar across all STS score categories.
Similarly, results from a subset of 257 “real-world” patients enrolled in the Evolut R FORWARD Study with an STS PROM < 3 percent demonstrated excellent clinical outcomes, with a low rate of all-cause mortality (2 percent) and disabling stroke (1.2 percent) at 30-days post implant.
The CoreValve Evolut TAVR platform consists of the CoreValve, CoreValve Evolut R and the Evolut PRO systems, which are available for use in the United States and Europe for severe aortic stenosis patients at an intermediate surgical risk or greater.
Watch the VIDEO "TAVR Stands Equal to Surgical Valve Replacement" — an interview with Michael Reardon, M.D.
Related Content
TCT 2017 Late-breaking Clinical Trial Presentations
References:


 November 14, 2025
November 14, 2025 









