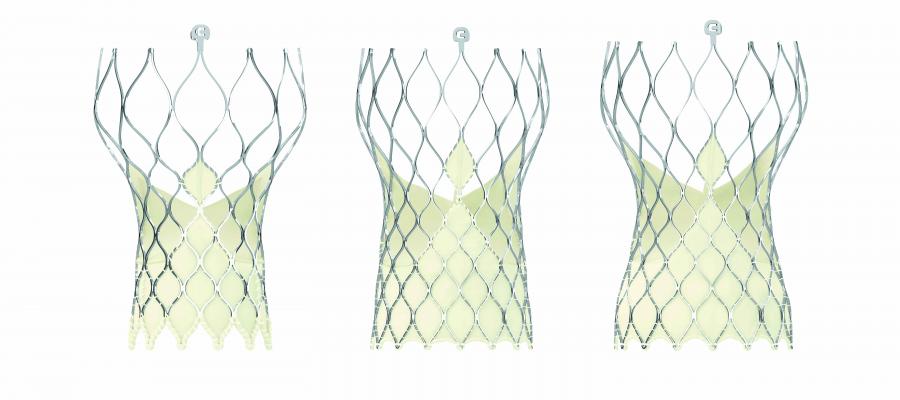
October 31, 2016 — Medtronic plc presented new positive data from two large registries aimed at evaluating 30-day clinical performance outcomes for the self-expanding, recapturable and repositionable CoreValve Evolut R System in “real-world” severe aortic stenosis patient populations at the Transcatheter Cardiovascular Therapeutics (TCT) Annual Meeting.
Evolut R FORWARD Study
Positive early clinical results from the first 300 patients enrolled in the FORWARD study—a global, single-arm, prospective study at 60 centers across Europe, Australia, the Middle East, Africa, Latin America and Canada— marks the first time that the self-expanding Evolut R System was evaluated in routine clinical practice on a global scale. Designed to confirm the results achieved in the Evolut R CE Study for patients with severe aortic stenosis, the FORWARD study demonstrated an exceptionally high survival (98 percent) and a low rate of all stroke (3 percent) at 30-days post-implant.
The Evolut R FORWARD study also showed improved hemodynamic performance (by mean aortic valve gradient, a measure of blood flow through the valve) from 42.5 ± 17.7 mm Hg at baseline to 8.7 ±6.9 mm Hg at discharge. Additionally, there was a low rate of major vascular complications (2.7 percent) and no reports of valve thrombosis at 30-days.
“We’re encouraged by the initial 30-day outcomes of the FORWARD study, which further showcase the advantages of the recapturable and repositionable capabilities of the Evolut R System,” said Prof. Eberhard Grube, M.D., director of the Structural Heart Program at University Hospital in Bonn, Germany, and co-principal investigator of the FORWARD Study. “As patient follow-up continues at one, two and three-years post-implant, we look forward to seeing how the features of the Evolut R System can address everyday clinical needs in various severe aortic stenosis patient scenarios worldwide.”
STS/ACC TVT Registry Data
New data from the Society of Thoracic Surgeons and American College of Cardiology (STS/ACC) Transcatheter Valve Therapy (TVT) Registry also demonstrated successful real-world outcomes achieved with the Evolut R System.
In the analysis of 9,616 patients implanted with Medtronic self-expanding transcatheter aortic valve replacement (TAVR) systems, Evolut R demonstrated high survival (96.3 percent) and low rates of all stroke (3.1 percent) at 30 days, with successful valve implantation (99 percent). Patients in this analysis also experienced improved hemodynamic performance (mean gradient: 43.7 ± 15.4 mm Hg at baseline to 8.6 ± 5.5 mm Hg at discharge) and low rates of major vascular complications (1.5 percent). Additionally, post-procedure hospital stays were a median of four days for Evolut R and the majority of patients treated with Evolut R (75.7 percent) returned home following discharge, as opposed to a nursing home or other rehabilitation facility.
The CoreValve Evolut R System is available in Europe and other countries that recognize the CE Mark and was approved for commercial use in the United States in 2015.
For more information: www.medtronic.com


 November 14, 2025
November 14, 2025 









