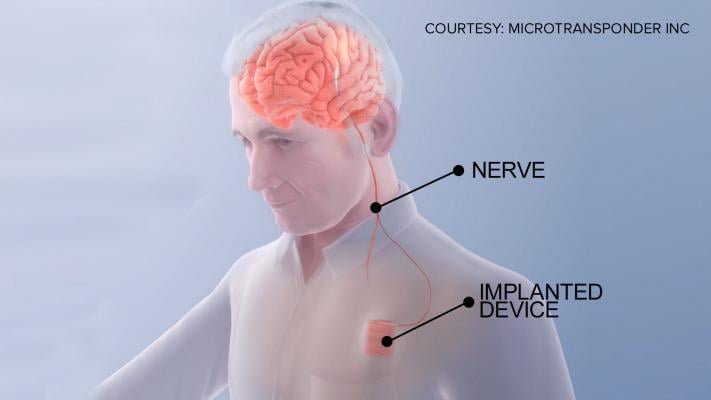
A new clinical trial at The Ohio State University Wexner Medical Center is examining an implanted device that uses vagus nerve stimulation to help stroke patients regain motor function. The Vivistim device is similar to a pacemaker, which uses leads to electrically stimulate the brain.
May 9, 2018 — Stroke rehabilitation specialists at the The Ohio State University Wexner Medical Center are among the first in the world involved in a new clinical trial that uses vagus nerve stimulation to improve rehabilitation for stroke patients. Patients are implanted with an electrical device in their chest wall called a vagus nerve stimulator that connects to the vagus nerve in the neck.
Strokes are the leading cause of long-term disability in the United States, and they can happen at any age. For many, a stroke affects the mobility of their arms and hands, making it difficult to perform routine, everyday tasks. But experts are studying a new approach to stroke rehabilitation that could help patients recover their motor skills sooner.
The MicroTransponder Vivistim device is used to “rewire” circuits in the brain associated with certain motor functions. During rehabilitation therapy sessions, when a patient correctly performs an exercise, the device stimulates the vagus nerve — which signals the brain to remember that movement.
“The goal is to see if we can improve motor recovery in people who have what is, in effect, a brain pacemaker implanted in their body,” said Marcie Bockbrader, M.D., Ph.D., research physciatrist for the Neurological Institute at The Ohio State University Wexner Medical Center. “The idea is to combine this brain pacing with normal rehab and see if patients who’ve been through all of the other usual therapies after a stroke can get even better.”
This new approach has previously been shown to benefit approximately 85 percent of the people who received the nerve stimulation. Bockbrader says the nerve stimulation is like turning on a switch, making the patient’s brain more receptive to therapy.
Watch the VIDEO "Pacemaker For Stroke Recovery Tested by The Ohio State University."
For more information: wexnermedical.osu.edu



 January 29, 2026
January 29, 2026 









