May 4, 2011 -- Two new clinical studies will use the Carillon Mitral Contour System, an investigational device for percutaneous treatment of functional mitral regurgitation (FMR). The Carillon system, made by Cardiac Dimensions Inc., is a minimally invasive device designed to repair the heart's mitral valve and reduce FMR, a disorder that affects most of the estimated 5 million people in the United States and more than 20 million people worldwide suffering from heart failure.
The INTEGRAL Trial will assess safety and efficacy of the Carillon device at one, three, six and 12 months by evaluating reduction in FMR, heart size and corresponding improvements in exercise capacity. The TITAN II study of the Carillon is an extension of the company's recent TITAN trial.
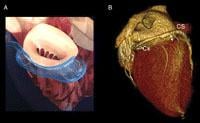
The device combines a proprietary implantable device and delivery system. The implant consists of a shaping ribbon between distal and proximal anchors. It is delivered percutaneously via jugular vein access under fluoroscopic guidance. The implant is designed to be positioned, adjusted and gently anchored in the coronary sinus to reshape the annulus around the mitral valve, thereby reducing mitral regurgitation. Multiple clinical studies have shown that the reduction in mitral regurgitation is associated with improvements in other key functional parameters including New York Heart Association (NYHA) class, six minute walk distance, and quality of life.
The follow-up results from previous clinical studies of the system have prompted the company to expand its clinical trial efforts and prepare for European commercialization of the device in the near future.
The company also announced that Adrian Ebner, M.D., the principal investigator of the INTEGRAL trial, successfully performed the first implant using the Carillon system. Ebner performed the procedure at the Italian Hospital in Asuncion, Paraguay on a 32-year-old man with moderate mitral regurgitation (MR) caused by his dilated cardiomyopathy.
"The patient had been in the hospital for several weeks prior to the procedure, but after the Carillon procedure we saw an immediate improvement in MR," Ebner said. "The patient also indicated improvement in his heart failure symptoms as early as the following day."
For more information: www.cardiacdimensions.com


 January 05, 2026
January 05, 2026 









