January 27, 2010 – A German study enrolled its first 20 patients to investigate the utility of muscular counter pulsation (MCP) in patients with early-stage heart failure.
Cardiola AG is collaborating with The Heart and Diabetes Center North Rhine-Westphalia, Ruhr University of Bochum, Bad Oeyhausen, Germany, in a study of the company’s CE-marked m.pulse system. MCP was previously only available in a clinical setting.
Under the supervision of the clinical investigating team, early-stage heart failure patients will use the m.pulse system at home on a prescribed schedule and will be evaluated to determine the impact of the treatment on their symptoms.
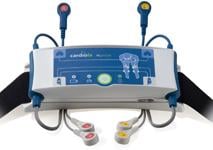
Battery-powered m.pulse, the size of a cell phone that the patient attaches to his belt for about 45 minutes per treatment, is synchronized to his cardiac cycle to stimulate the muscles of the calves and thighs to make them contract in the resting phase of the heart. The MCP action results in increased blood flow to the heart muscle while decreasing the heart’s workload.
For more information: www.cardiola.com


 January 05, 2026
January 05, 2026 









