
February 24, 2021 — Occlutech announced the completion of patient enrollment in the PRELIEVE trial, pilot study to assess safety and efficacy of the novel Atrial Flow Regulator (AFR) in heart failure (HF) patients.
PRELIEVE is a prospective, multicenter, open-label, non-randomized pilot study that evaluates the results of AFR implantation in patients with either heart failure with preserved ejection fraction (HFpEF) or heart failure with reduced ejection fraction (HFrEF).
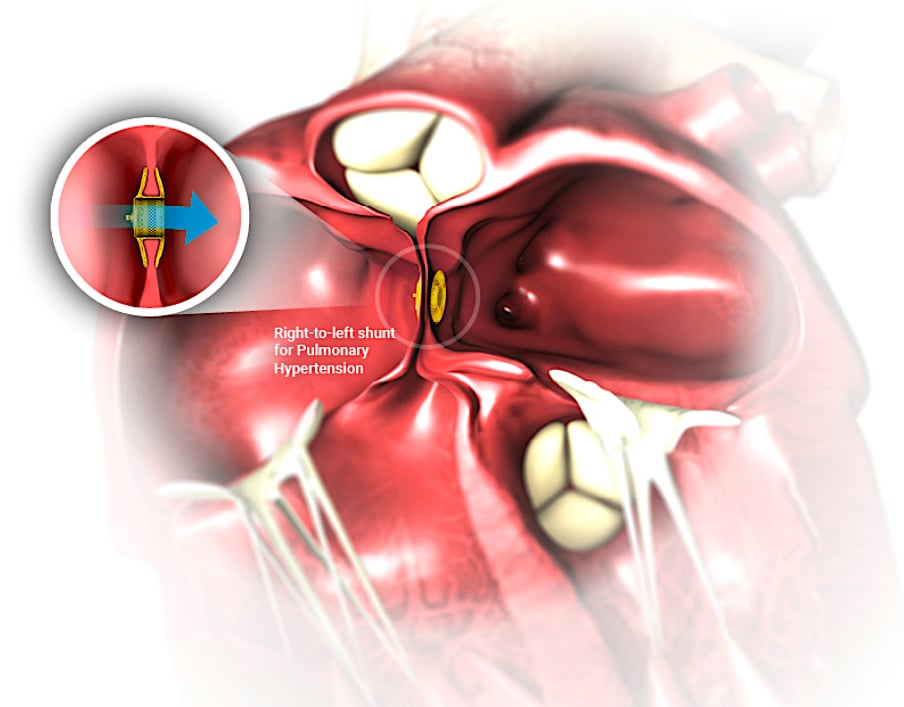
The Occlutech implantable Atrial Flow Regulator (AFR) maintains an interatrial shunt with a predetermined diameter allowing for controlled blood flow from the left to the right atrium enabling the left atrium to decompress and lower left atrial pressure. This reduced left atrial pressure reduces heart failure symptoms and improves exercise tolerance.
HF affects millions of people around the globe and has a big impact on mortality and healthcare expenditure. Even with the best medical therapy, many patients experience persistent symptoms and low quality of life as a result of elevated left atrial pressure.
AFR is a minimally invasive cardiac implant that is designed to maintain a permanent interatrial communication and allows controlled blood flow from overloaded left atrium to the lower pressure right atrium.
"The completion of the enrollment is another important milestone for Occlutech and we are excited to support this therapy option for heart failure patients with our clinical activities." said Sabine Bois, CEO of the Occlutech Group.
Occlutech is one of the leading companies in its field, with several major products including state-of-the-art PFO occluders, ASD occluders among others. Occlutech has sales of congenital and structural heart products in over 80 countries and maintains manufacturing and R&D facilities in Jena, Germany and Istanbul, Turkey. Occlutech has developed many novel products and technologies to improve treatment of patients in these and related areas.
The AFR is not approved in the United States. Product availability is subject to local regulatory clearance. The AFR is under clinical investigation for use in patients with pulmonary arterial hypertension and use in these patients is limited by applicable national laws.
For more information: www.occlutech.com
Occlutech's Atrial Flow Regulator for Heart Failure Receives FDA Breakthrough Device Designation


 January 05, 2026
January 05, 2026 









