October 4, 2018 — The Ohio State University Wexner Medical Center is first in the country to test a new medical device designed to help patients with advanced heart failure. Cardiologists here performed the first two implants and the patients went home the next day.
“This is the latest in more than 200 active research trials that Ohio State has available for patients with heart and vascular disease,” said K. Craig Kent, M.D., dean of The Ohio State University College of Medicine. “Our physician researchers are truly committed to identifying new ways to treat and prevent cardiovascular disease.”
Heart failure affects about 6 million Americans and there are about 800,000 new cases each year. When the disease is advanced, the failing heart can’t effectively pump blood out to the body. The fluids build up and cause increased pressure inside the heart and into the lungs, causing shortness of breath.
“Despite recent advancements in medical and device therapies, a large percentage of patients remain symptomatic. There is hope that this device will offer another option to relieve left atrial pressure, which is the cause of heart failure symptoms and hospitalizations for both systolic and diastolic heart failure,” said Garrie Haas, M.D., Ohio State’s principal investigator on the study and a heart failure cardiologist.
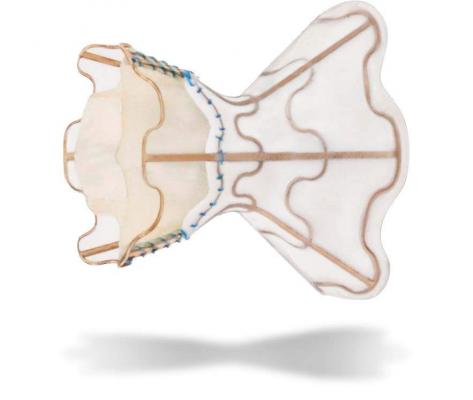
The device is an investigational interatrial shunt made by V-Wave Ltd. Doctors implant the hourglass-shaped device by threading it through a catheter placed in the groin.
“The shunt is placed between the upper two chambers of the heart and offers a pop off valve. Patients are minimally sedated and recovery is expected within a few hours," said Scott Lilly, M.D., Ph.D., director of the Structural Heart Disease Program and the interventional cardiologist who implanted the first two devices.
The shunt keeps the path open and diverts some of the built up blood from the high pressure left atrial chamber to the lower pressure right atrial chamber. The trial is to determine if shunting can reduce symptoms, improve heart function and reduce hospitalizations.
The clinical trial will include patients with advanced heart failure (New York Heart Association Class III or ambulatory Class IV) and shortness of breath with little exertion or trouble breathing when lying down. Additionally, candidates will have been hospitalized for heart failure within the last year. The first few patients at each research site will receive the shunt implant. The rest will be randomly assigned to receive the device or conventional therapy. In all, the trial will enroll about 500 patients at up to 75 sites around the world.
For more information: www.vwavemedical.com


 January 05, 2026
January 05, 2026 









