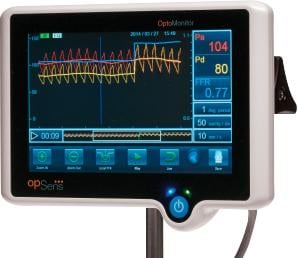
Image courtesy of Opsens
January 14, 2015 — Opsens Inc. announced the first use of its Fractional Flow Reserve (FFR) products in Europe by the founders of FFR, namely Bernard De Bruyne, M.D. at the Cardiovascular Center Aalst, Belgium and by professor Nico Pijls, at the Catharina Hospital, Eindhoven the Netherlands. The OptoWire and OptoMonitor are Opsens’ products for FFR measurements to optimize the diagnosis and guide the therapy in patients with coronary heart disease.
"The arrival of an optical FFR guidewire such as the OptoWire on the market is positive for interventional cardiologists who need to obtain reliable FFR measurements. It allowed me to appreciate its zero drift performance during all cases performed while also acknowledging the constant connection reliability as well as its support during percutaneous coronary intervention", said Bernard De Bruyne, M.D., from the Cardiovascular Center Aalst.
The OptoWire provides intra-coronary blood pressure measurements with optical pressure guidewire technologies. It is immune to adverse effects related to blood contact, and allows easy connectivity that leads to reliable FFR measurements in extended conditions of usage. The product is designed to provide cardiologists with a guidewire delivering optimized performances to navigate coronary arteries and reach blockages. Based on industry sources, the FFR market represented more than $250 million in U.S. sales in 2013 and is expected to reach $1 billion in the medium-term.
For more information: www.opsens.com


 November 14, 2025
November 14, 2025 









