September 21, 2010 – The first patients have been enrolled in a trial testing a new treatment for refractory angina. The COSIRA (Coronary Sinus Reducer for Treatment of Refractory Angina) trial is designed to assess the clinical efficacy of the Neovasc Reducer. The goal is to minimize angina symptoms after six months.
The study, which is being conducted under the supervision of Dr. Stefan Verheye at the Antwerp Cardiovascular Institute/ZNA Middleheim in Belgium, is expected to enroll up to 124 patients.
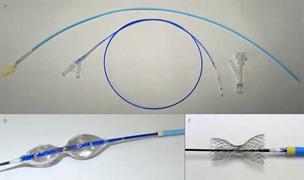
The Neovasc Reducer is designed to treat heart disease patients suffering from refractory angina, a painful and debilitating condition that occurs when the coronary arteries deliver an inadequate supply of blood to the heart muscle. It currently affects more than 2 million patients worldwide, who typically lead severely restricted lives.
The device is implanted in the coronary sinus vein using a minimally invasive percutaneous procedure that is similar to implanting a coronary stent. It is intended to provide relief by modulating blood flow in the coronary sinus and thereby increasing perfusion of oxygenated blood to areas of the heart muscle where it is needed most.
The COSIRA trial is also initiating patient enrollment at Ziekenhuis Oost-Limburg Hospital in Genk, Belgium; Montreal Heart Institute and the University of Ottawa Heart Institute in Canada; and Maastricht University Medical Centre and Utrecht University Medical Center in the Netherlands. Enrollment is expected to be completed by mid-2011.
“We are optimistic that the clinical data from COSIRA will support the excellent results we have seen in our previous clinical studies and confirm that the Reducer offers a relatively straightforward and viable long-term treatment option for patients with refractory angina, who currently lack alternatives for relieving their symptoms and improving their quality of life,” said Prof. Shmuel Banai, M.D., Neovasc Medical Director. "We are very pleased to have attracted such a strong group of clinical investigators from high-quality cardiovascular medical centers for this trial and look forward to sharing initial data from COSIRA in the coming year.”
For more information please visit: www.neovasc.com


 January 05, 2026
January 05, 2026 









