May 19, 2011 - Neovasc Inc., a developer of novel technologies used to treat vascular disease, reported positive six-month follow-up data for a patient with severe refractory angina who received the Neovasc Reducer product in a “live case” procedure broadcast during the 22nd annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium in September 2010. The data, which showed a marked improvement in the patient’s angina symptoms, was presented today at EuroPCR, a leading European cardiovascular conference.
Dr. Stefan Verheye of the Antwerp Cardiovascular Institute / ZNA Middelheim had successfully implanted the Neovasc Reducer during the TCT “live case” demonstration. He stated, “The results seen to date in this patient are excellent and suggest that implantation of the Reducer device has the potential to significantly improve the quality of life of patients with refractory angina. I am hopeful that these types of positive results will also be seen in the patients currently being enrolled in the COSIRA clinical trial, which is designed to rigorously assess the utility of the Reducer in treating refractory angina.”
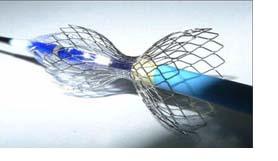
The Neovasc Reducer is a novel device designed to treat patients who suffer from refractory angina, a painful and debilitating condition that occurs when the coronary arteries deliver an inadequate supply of blood to the heart muscle. The incidence of refractory angina is growing, yet current treatment options are limited. The Reducer is implanted in the coronary sinus vein using minimally invasive techniques. By altering blood flow in the coronary sinus, the Reducer acts to increase the perfusion of oxygenated blood to certain areas of the heart muscle, thereby reducing the pain and disability caused by the condition.
The results presented at EuroPCR showed a marked improvement in the patient’s angina symptoms six months following implantation of the Reducer device. At the time of implantation, in spite of multiple prior revascularizations, the patient was classified as having CCS Class III angina, which included experiencing substantial limitations due to angina pain interfering with everyday activities, such as walking a short distance or climbing a flight of stairs. Since implantation of the Reducer device, her angina classification has improved to CCS Class I, in which patients typically experience angina symptoms only with strenuous, rapid, or prolonged exertion.
In a video interview conducted last week, the patient reported that she no longer has to take the majority of her angina medications and is now riding her bicycle for long periods each day without significant angina pain. Her improvement has also been documented using quantitative imaging methods. At baseline the patient demonstrated significant ischemia of the heart muscle in certain areas around the left ventricle as measured using echo dobutamine stress testing. At follow-up testing, these areas appeared substantially normal, with no significant ischemia observed.
Neovasc is presently enrolling patients in the COSIRA (Coronary Sinus Reducer for Treatment of Refractory Angina) trial. COSIRA is a multicenter, sham-controlled, randomized, double-blinded study. It has been designed to provide controlled, statistically significant data to further demonstrate the efficacy of the Reducer product to support regulatory applications and marketing for the millions of refractory angina patients who could potentially benefit.
For more information, visit: www.neovasc.com


 January 05, 2026
January 05, 2026 









