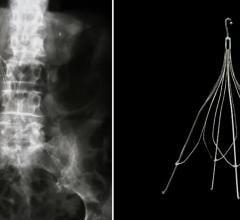
Possis Medical, Inc. has closed a $2.5 million equity investment in Rafael Medical Technologies, Inc., which gives Possis 15 percent ownership in Rafael Medical and a three-year option to purchase the company.
The deal does hand Possis an obligation to provide up to $1.5 million of secured debt financing but also offers Possis one seat on Rafael's board of directors. Rafael Medical is developing its proprietary SafeFlo inferior vena cava (IVC) filter. It is estimated that by 2010, the U.S. market for IVC filters will total nearly $200 million. Rafael Medical is conducting a clinical trial of the SafeFlo filter at multiple sites in the U.S. and Europe; a 510(k) submission to the FDA is expected within 12 to 18 months.
The IVC is the large vein that carries de-oxygenated blood from the lower body back to the heart and then to the lungs for re-oxygenation. In certain individuals, the formation of blood clots in the deep veins of the legs, a condition known as deep vein thrombosis (DVT), can lead to the migration of blood clots to the lungs causing a pulmonary embolism (PE), an often fatal condition. The U.S. patented SafeFlo IVC filter is a minimally invasive and retrievable filter designed to prevent the migration of thrombus to the lungs and the potentially devastating effects of PE.
The purchase option may be exercised by Possis at any time, at the discretion of Possis, prior to the earlier of 60 days after Rafael receives U.S. FDA marketing clearance, or November 29, 2009. If Possis determines to exercise the option, it will pay Rafael Medical shareholders an initial purchase price of $12 million in cash, less any debt outstanding, and will be obligated to make earn-out payments based on a multiple of revenue generated from the SafeFlo IVC filter during the three years commencing six months after closing of the transaction. Total payments, including the $2.5 million initial equity investment, will not exceed $54 million.


 July 27, 2021
July 27, 2021