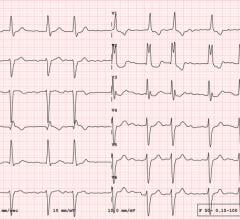
May 8, 2020 — New clinical trial reveals the first-in-human results for paroxysmal or persistent atrial fibrillation (AF) treated with a novel energy source – pulsed field ablation (PFA). The study demonstrates the safety and efficiency benefits of using PFA to target abnormal heart rhythms through pulmonary vein isolation (PVI). Findings of the PULSED AF study were presented today as a late-breaking clinical trial as part of Heart Rhythm Society (HRS) 2020 Science.
PFA technology also is called electroporation, because it causes temporary pore-like openings in the cell walls, which aids in cell death and more complete ablation procedures.
Ablation is a common treatment option for patients with AF, with an estimated 75,000 ablation procedures performed each year in the United States alone.[1] Ablation involves using PVI to ablate, or eliminate the cause of irregular heartbeats. For the past 25 years, the procedure has been performed using thermal energy sources such as radiofrequency (RF) or recent cryoballoon energy. Thermal energy can cause collateral damage to organs and nerves surrounding the heart such as the lungs, esophagus and chest cavity, while pulsed electrical fields target cardiac tissue.
PULSED AF is a non-randomized, prospective, multi-center, global, pre-market clinical study performed in Australia, Canada, United States and Europe. The study evaluates the Medtronic PulseSelect system, a PFA system that delivers bipolar, biphasic pulsed electric fields through a circular multi-electrode array catheter to perform PVI. The patients included were undergoing first-time ablation for either paroxysmal or persistent AF (less than one year). The study endpoints include AF recurrence >30 seconds and procedural safety. AF monitoring is being performed by weekly trans-telephonic transmission and intermittent Holters at six and 12 months. Patients will ultimately be followed for 12-months post-ablation.
Results of the PULSED AF study suggest that PFA delivers ablation as effective as RF ablation, while providing a safer and more efficient procedure. Results show acute electrical isolation was achieved in 100 percent of patients to-date and there were no tamponades, strokes or phrenic nerve injuries. Upon conclusion, the study will report the rate of arrhythmia-free survival at 12 months, and pre-specified secondary and ancillary endpoints, including: procedural outcomes, quality of life and arrhythmic symptoms.
“As ablation technology evolves, we saw the opportunity to improve upon the procedure to treat this growing patient population,” said lead author Atul Verma, M.D., FHRS, Southlake Regional Health Center, Toronto, Ontario, Canada. “For the first time, we are able to see positive, in-human
benefits of using a unique, nonthermal energy source to address safety concerns associated with ablation. We look forward to seeing how this technological innovation will move the electrophysiology field forward in advancing this crucial solution and reducing the impact of persisting AF.”
The authors of this study point to a future presentation of full, 12-month patient follow-ups results. This trial will be followed by FDA submission for an international, pivotal trial to provide additional clinical data.
Read more in the January 2020 article Medtronic FDA Trial Evaluates Pulsed Electric Fields to Treat Atrial Fibrillation.
Read another pulsed field study presented at HRS 2020 — Pulsed Field Ablation Successfully Treats Atrial Fibrillation
Find links to all the Heart Rhythm Society 2020 Late-Breaking Clinical Trials in Electrophysiology
Related Pulse Electric Field Ablation Content:
Medtronic to Acquire Affera to Expand Cardiac Ablation Portfolio
Boston Scientific Invests in Pulsed Field Ablation Technology To Improve Atrial Fibrillation Therapy
Medtronic FDA Trial Evaluates Pulsed Electric Fields to Treat Atrial Fibrillation
First Patients Treated with Galaxy Medical Centauri Pulsed Electric Field Cardiac Ablation System
References:


 July 30, 2024
July 30, 2024